| Title: | 3-Pyrimidin-4-yl-oxazolidin-2-ones as inhibitors of mutant IDH | ||
| Patent Application Number: | WO 2014/141153 A1 | Publication Date: | 18 September 2014 |
| Priority Application: | US 61/782,211 | Priority Date: | 14 March 2013 |
| Inventors: | Cho, Y. S.; Level, J. R.; Liu, G.; Shultz, M. D.; Van Der Plas, S. C. | ||
| Assignee Company: | Novartis AG; Lichtstrasse 35, CH-4056 Basel (CH) | ||
| Disease Area: | Cell proliferation disorders, such as cancer | Biological Target: | Mutant isocitrate dehydrogenase (IDH) |
| Summary: | The invention in this patent application relates to 3-pyrimidinyl-4-yl-oxazolidin-2-one derivatives represented generally by Formula (I). The compounds of the invention are inhibitors of mutant isocitrate dehydrogenase (IDH) and may be useful for the treatment of cell proliferation disorders such as cancer. | ||
| lsocitrate dehydrogenases (IDHs) are oxidoreductases of the EC 1.1.1.42 enzyme class that constitute a key family of enzymes found in cellular metabolism. Two of the IDH isoforms, IDH1 (cytosolic) and IDH2 (mitochondrial), carry out the oxidative decarboxylation of isocitrate with the mediation of NADP+/NAD+ and metal cations to form α-ketoglutarate, CO2, and NADPH/NADH. | |||
| Several cancer types are associated with mutations in IDH1 and IDH2. Examples of these cancers include glioma, glioblastoma multiforme, paraganglioma, supratentorial primordial neuroectodermal tumors, acute myeloid leukemia (AML), prostate cancer, thyroid cancer, colon cancer, chondrosarcoma, cholangiocarcinoma, peripheral T-cell lymphoma, and melanoma. The mutant forms of IDH display a neomorphic activity that causes the stereospecific reduction of the keto group of α-ketoglutarate to form the (R)-enantiomer of 2-hydroxyglutarate (labeled D- or R-2-HG). While normal cells contain low levels of 2-HG, cells that harbor the mutant forms of IDH1 or IDH2 show significantly high levels of 2-HG. For example, high levels of 2-HG are associated with tumorigenesis and have been detected in the plasma of patients with mutant IDH containing AML. Mutant IDH2 is associated with a rare neurometabolic disorder called d-2-hydroxyglutaric aciduria type II (d-2-HGA type II). Patients affected by this disorder show high levels of (R)-2-HG in their urine, plasma, and cerebrospinal fluid. Also patients with the rare Oilier disease and Mafucci syndrome (which predispose to cartilaginous tumors) have been shown to be somatically mosaic for IDH1 and IDH2 mutations and exhibit high levels of (R)-2-HG. | |||
| Thus, the compounds described in this patent application, which are inhibitors of mutant IDH having a neomorphic activity, may potentially provide treatments for the diseases and disorders associated with these mutant enzymes including, but not limited to, cell proliferation disorders, such as cancer. | |||
| Important Compound Classes: | 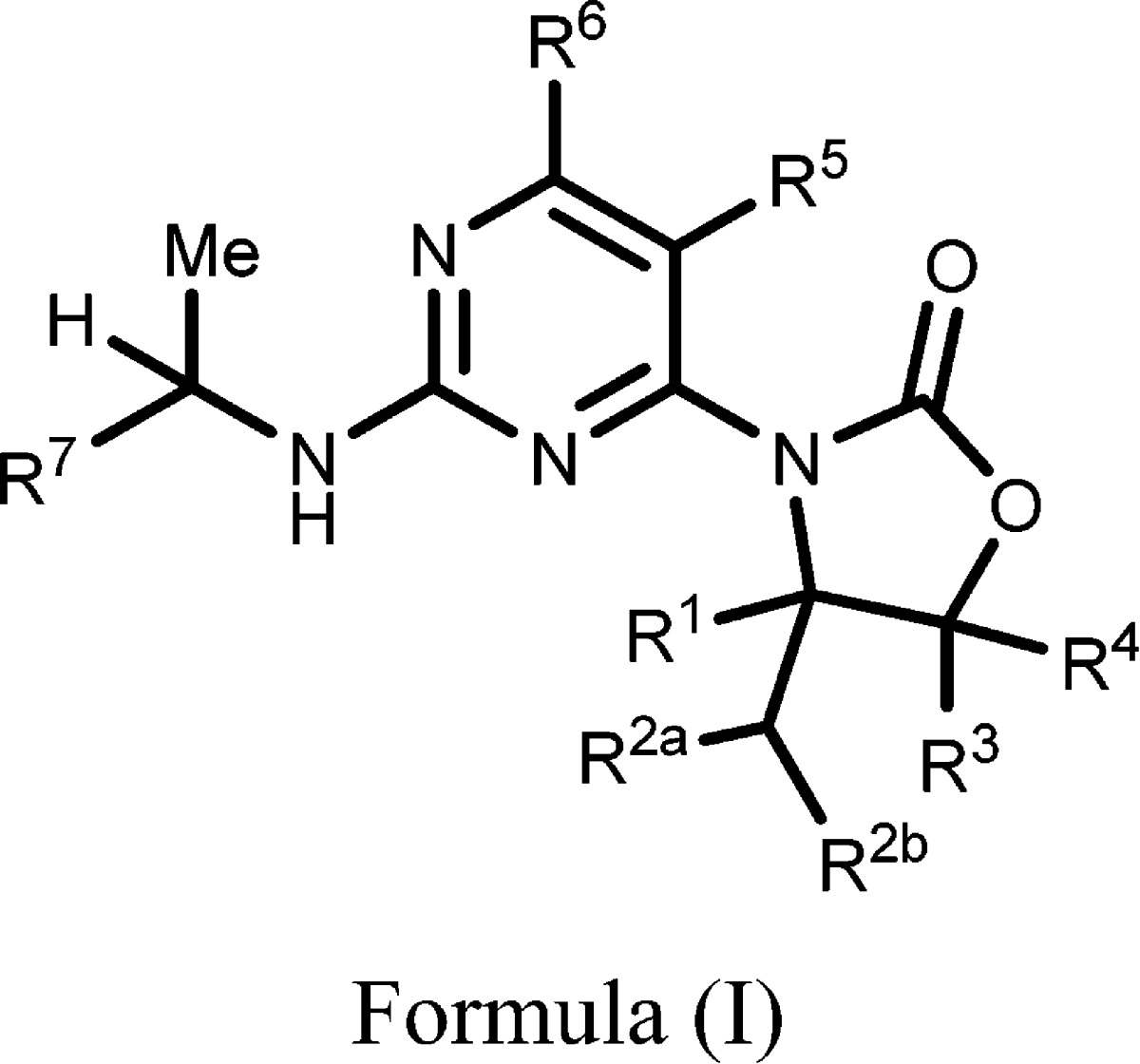 |
||
| Key Structures: | The inventors listed the structures of 69 structures representing formula (I); the following four compounds are representative examples: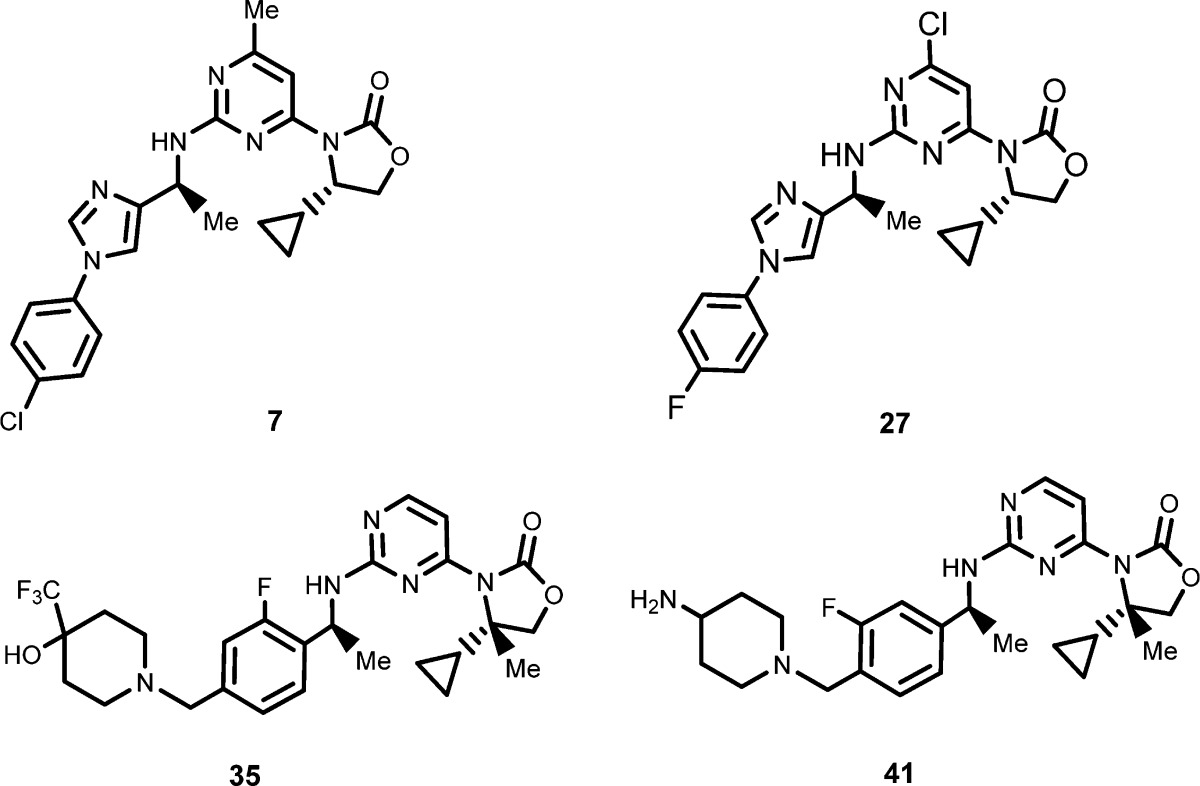
|
||
| Biological Assay: |
|
||
| Biological Data: | The biological data obtained from the above two assays were listed for most examples. The following table contains the IC50 data for the above four representative compounds: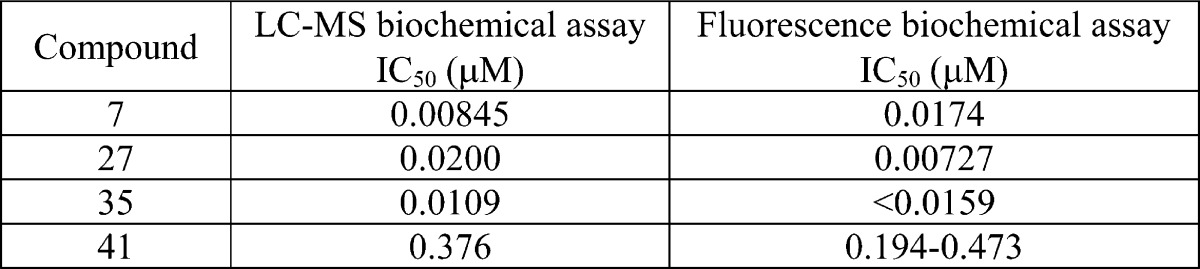
|
||
| Recent Review Articles: | 1. Levis M.Blood 2013, 122 (16), 2770–2771. | ||
| 2. Garrett-Bakelman F. E.; Melnick A. M.. Cell Res. 2013, 23 (8), 975–977. | |||
| 3. Pirozzi C. J.; Reitman Z. J.; Yan H.. Cancer Cell 2013, 23 (5), 570–572. | |||
The authors declare no competing financial interest.


