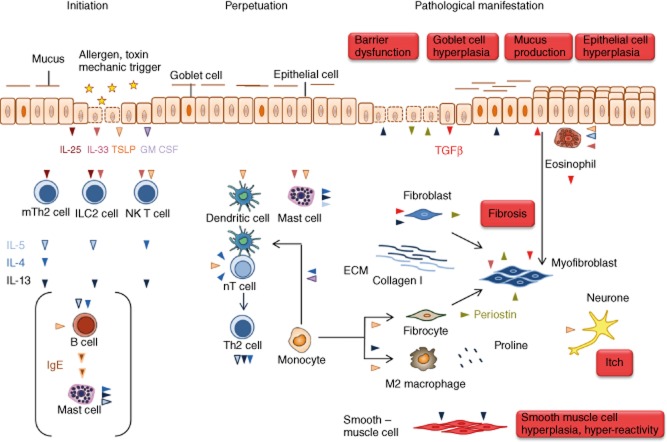
Fig. 1.
The route to pathologies in T helper type 2 (Th2)-characterized chronic inflammatory diseases. The inflammatory cascade is initiated by the cytokines interleukin (IL)-25, IL-33 and thymic stromal lymphopoietin (TSLP) released from epithelial cells experiencing damage by allergens with proteolytic activity or toxins. The first addresses of these signals are MutT homologue 2 (mTh2) cells, innate lymphoid cells (ILCs) and invariant natural killer T2 (iNK T2) cells. These cells secrete the typical Th2 cytokines IL-4, IL-5 and IL-13 responsible for perpetuation of the inflammation and for pathological changes. TSLP also activates dendritic cells to enforce the differentiation of naive T cells to Th2 cells and thus further promotes the Th2 inflammatory process. In addition, TSLP activates mast cells which, in turn, release IL-4, IL5 and IL-13. This axis might be responsible for mast cell activation in the absence of immunoglobulin (Ig)E as it is sometimes observed in atopic dermatitis and is common in ulcerative colitis (UC). IL-4 and granulocye–macrophage colony-stimulating factor (GM-CSF) induce the differentiation of monocytes to dendritic cells which instruct more naive T cells to become Th2 cells. In concert with periostin, IL-13 induces the differentiation of monocytes to alternatively activated macrophages which release components of the extracellular matrix. Periostin also promotes the differentiation of monocytes to fibrocytes which are considered as crucial for the manifestation of fibrosis. Fibrosis is the pathological phenotype shared by all three diseases and the outcome of the activity of various cell types. IL-5 activates eosionohils and TSLP stimulate eosinophils to secrete transforming growth factor (TGF)-β, an important cytokine to induce fibrosis by stimulating the secretion of extracellular matrix proteins from myofibroblasts. Upon interaction of eosionophils with epithelial cells, epithelial cells undergo epithelial mesenchymal transition and turn into myofibroblasts. In addition, resident fibroblasts and monocyte-derived fibrocytes become myofibroblasts. Although the pathologies in all three diseases differ according to the affected organ, it is obvious that epithelial cells are simultaneously activator and target of the inflammatory response. They secrete periostin in response to exposure to IL-13, which induces barrier dysfunction and respond to to periostin by secreting TGF-β. There are some differences in all three diseases which might reflect different molecular mechanisms underlying the disease. In asthma, IL-4 and IL-5 in concert with periostin promote the clonal expansion and maturation of B cells and induces the IgM/IgE switch. Released IgE activate mast cells to secrete IL-4, IL-5 and IL-13 and histamines and chemokines which promote the influx of inflammatory cells into the tissue. IL-13 induces goblet cell hyperplasia resulting in increased mucus production and smooth muscle cell hyperplasia and hyperactivity which are prominent pathological features of asthma. In atopic dermatitis, IgE release signifies the disease in many cases; however, it does not seem to be a prerequisite for the manifestation of the disease. In atopic dermatitis, IL-13 induces epithelial hyperplasia resulting in the thickening of the skin, and TSLP is most probably the cytokine responsible for itch. Colitis is characterized by severe epithelial damage responsible for diarrhoea and extensive fibrosis, which accounts for the loss of function of the colon. IL-13 is considered the key effector cytokine responsible for barrier dysfunction of the gut epithelia and fibrosis. Another specificity of UC is that the colon hosts the microflora, which has no contact with the apical side of the gut epithelia in a healthy condition, but which can invade the tissue when the barrier is defective.