FIGURE 5.
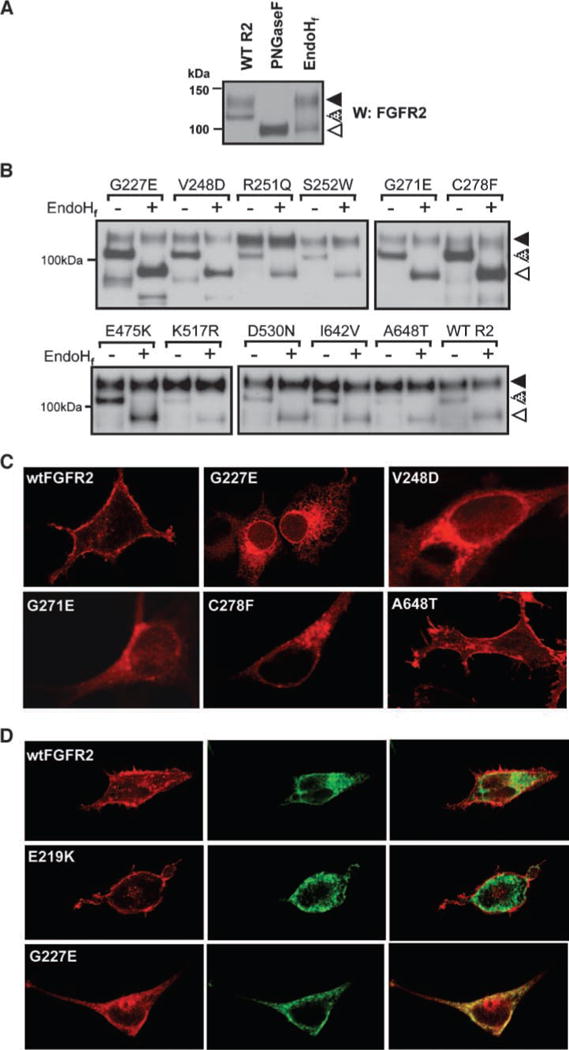
Mutations in FGFR2 impair receptor processing and localization. A. Confirmation of posttranslational glycosylation of wild-type FGFR2 by PNGase digestion of both the 130-kDa band (solid arrow) and 110-kDa band (stippled arrow) to a single 98-kDa band (open arrow) in HEK293 cells. Sensitivity to digestion with EndoHf of only the 110-kDa band indicates this 110-kDa band to be an immature, partially processed receptor predominantly present in the ER and early Golgi. B. EndoHf digestion of FGFR2 in HEK293 lysates transiently transfected with wild-type FGFR2 or mutant FGFR2. Densitometry analysis revealed that the EndoH-resistant 130-kDa band comprises ~60% of the wild-type FGFR2 but only 30% of G227E, V248D, G271E, and C278F mutants. C. Localization of wild-type and mutated FGFR2 following transient transfection into HEK293 cells and immunofluorescent staining. Impaired receptor trafficking is evident for G227E, V248D, G271E, and C278F compared with trafficking of the wild-type receptor to the cell surface. D. Immunofluorescent analysis of the colocalization of FGFR2 (red, left) and the ER-resident marker protein disulfide isomerase (green, middle) confirms the overall retention of the G227E mutant receptor in the ER compartment in HEK293 cells (yellow, right) when compared with the cell surface localization of wildtype FGFR2 and E219K (mapping onto the known extracellular crystal structure predicted the latter mutation to have no effect on misfolding).
