Figure 2.
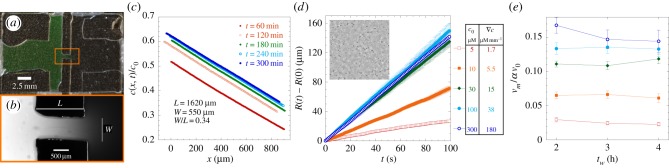
Experimental set-up and measurement of chemotactic fluxes. (a) Top view of the experimental sample chamber made of two reservoirs, containing attractant concentrations c = c0 and c = 0 (or here, fluorescein, appearing green). The reservoirs are in contact through a narrow channel in the centre (box region). (b) The channel (length L, width w) at higher magnification, observed under fluorescence microscopy (×10), 1 h after making the chamber. The fluorescence intensity is proportional to chemical concentration. (c) The chemical (fluorescein) concentration profile in the middle of the channel is linear and stabilized in tw = 2–3 h after filling the chamber. The concentration gradient ∇c and background concentration 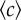 were calibrated as a function of c0, L and w (see the electronic supplementary material, figure S2 and text). (d) R(t) of E. coli bacteria at tw = 3 h, under different conditions but where the relative gradient
were calibrated as a function of c0, L and w (see the electronic supplementary material, figure S2 and text). (d) R(t) of E. coli bacteria at tw = 3 h, under different conditions but where the relative gradient 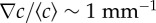 is held approximately constant. The measured trajectories are linear functions of time. The inset shows part of a typical image from a movie sequence, with sides of length 180 µm. (e) Measured chemotactic velocity as a function of tw for the same experiments as (d). The chemotactic velocity is nearly constant as a function of tw and increases as a function of
is held approximately constant. The measured trajectories are linear functions of time. The inset shows part of a typical image from a movie sequence, with sides of length 180 µm. (e) Measured chemotactic velocity as a function of tw for the same experiments as (d). The chemotactic velocity is nearly constant as a function of tw and increases as a function of 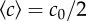 . The chemotactic velocities are normalized by the average swimming speed of the bacteria v0 and the fraction of swimmers α, as described in the main text. The key is common to (d,e).
. The chemotactic velocities are normalized by the average swimming speed of the bacteria v0 and the fraction of swimmers α, as described in the main text. The key is common to (d,e).
