Abstract
Seventeen herb, shrub and tree species of commercial and ecological importance in southern Africa were exposed at one location to ultraviolet‐B (UV‐B, 280–315 nm) radiation approx. 35 % above clear‐sky background (control). The aims were to assess how UV‐B affects canopy area, dry mass, and some biochemical and morphological properties of leaves, and to investigate whether differences between species are related to growth form of the plants. There was no pattern of response to UV‐B related to growth form. Leaves of trees had altered chlorophyll a and b, carotenoid and flavonoid concentrations, but those of shrubs or herbs did not. Non‐structural carbohydrates were unaffected. Smaller canopy areas and dry masses were observed under enhanced UV‐B, but these were not statistically different among growth forms. There was a general insensitivity of species to elevated UV‐B. Only five species had significantly altered leaf biochemical and morphological properties, canopy area and dry mass, the changes differing in magnitude. There was no consistent pattern of change in leaf thickness or biochemical composition with increased UV‐B. Correlation analyses did not support the view that growth is less negatively affected in species with thick leaves or in those where leaf thickness increases, or in species with naturally high leaf flavonoid contents or that are able to synthesize additional flavonoids in response to UV‐B enhancement. The analyses did not support the hypothesis that growth was inhibited by starch accumulation in leaves under elevated UV‐B. However, changes in leaf shape did correlate with canopy area and dry mass, showing the importance of photomorphogenetic changes caused by UV‐B which affect species’ performance. We conclude that generalizations on plant sensitivity to UV‐B based on growth form and functional type could be misleading, and that the great majority of economically important species of the region are likely to be insensitive to future UV‐B increases. Notable exceptions include the Colophospermum mopane tree ecotypes chota and leslie and the arable annual Vigna unguiculata, both of which are traditional sources of livelihood to rural African populations and of importance to African industry and agriculture.
Key words: African plant, carbohydrate, chlorophyll, flavonoid, growth, leaf area, morphology, plant growth form, ultraviolet‐B radiation, Colophospermum mopane, Virgilia oroboides, Leucadendron laureolum, Podalyria calyptrata, Protea burchellii, Cliffortia ruscifolia, Hermania saccilifera, Phylica pubescens, Aspalathus linearis, Barleria obtusa, Cyclopia genistoidesGlycine max, Phaseolus vulgaris, Vigna subterranea, Vigna unguiculata, Lupinus luteus, Vicia atropurpurea
INTRODUCTION
Depletion of stratospheric ozone has increased solar ultraviolet‐B (UV‐B, 290–315 nm) radiation at high‐ and mid‐latitudes (Madronich and de Gruijl, 1994) in both the Southern and Northern hemispheres (Seckmeyer et al., 1994; McKenzie et al., 1999). However, ozone destruction is more intense over the Southern hemisphere (Crutzen, 1992) with measured solar UV‐B fluxes up to 50 % greater than those at comparable latitudes in the Northern hemisphere (Seckmeyer et al., 1995). Consequently, increases in solar UV‐B may have a greater impact on plants in agricultural production and in natural ecosystems in the Southern than in the Northern hemisphere.
Studies examining effects of UV‐B on plants have mostly considered Northern‐hemisphere species, especially those of importance to local agriculture and forestry (Teramura, 1983; Caldwell, et al., 1995; Laakso and Huttunen, 1998). UV‐B radiation above ambient may inhibit plant growth, development and reproduction, and depress photosynthesis (Teramura and Sullivan, 1994; Rozema et al., 1997; Jansen et al., 1998). However, plant sensitivity to UV‐B differs between species (Teramura, 1983) and even varieties (Reed et al., 1992; Barnes et al., 1993; Correia et al., 1998). It is modified by plant growth rate (Eichhorn et al., 1993), developmental stage (Teramura and Sullivan, 1987), growth form (herbs cf. trees) and functional type (Gwynn‐Jones et al., 1999). Also, air temperature (Mark and Tevini, 1997), atmospheric carbon dioxide concentrations (Sullivan, 1997) and soil nitrogen (Hunt and McNeil, 1998; Correia et al., 2000), phosphorus (Murali and Teramura, 1985) and moisture (Sullivan and Teramura, 1990) contents may affect plant sensitivity to UV‐B.
Disparities in UV‐B sensitivity among species, varieties and groups of species of different growth form and functional type (Gwynn‐Jones et al., 1999) have mainly been attributed to the efficiency of foliage at screening metabolic processes (Day et al., 1992, 1994). One important predictor of this is the concentration of secondary metabolites in leaves (Day, 1993). These derivatives of the phenylpropanoid pathway include flavonoids, hydroxycinnamic acid derivatives and related phenolics, which absorb UV‐B radiation (Sheahan, 1996; Hoque and Remus, 1999). Negative correlations have consistently been observed between leaf phenylpropanoid concentrations and UV‐B transmittance in many taxa (Day, 1993), with different UV‐B absorption spectra apparent in whole‐leaf extracts of woody and herbaceous forms (Day et al., 1994). The UV‐B absorption, strong antioxidant (Dawar et al., 1998) and energy dissipating properties (Smith and Markham, 1998) of phenylpropanoids assist in limiting damage to the photosynthetic apparatus (Tevini et al., 1991) and to DNA (Stapleton and Walbot, 1994). This is highlighted by the hypersensitivity to UV‐B displayed by plant mutants deficient in the general phenylpropanoid or flavonoid pathway (Li et al., 1993). Also, increased production of various phenylpropanoids in response to UV‐B irradiation has been widely reported (Rozema et al., 1997) and associated with inter‐ and intraspecific differences in UV‐B sensitivity (Day et al., 1994).
Comparisons of UV‐B sensitivity among individual species or groups of species of different growth form or functional type are problematic (Gwynn‐Jones et al., 1999). Most studies have examined the responses of individual species and/or their varieties to UV‐B. These are often grown under unrealistic and unbalanced UV‐B, UV‐A and photosynthetically active radiation (Caldwell et al., 1994; Middleton and Teramura, 1994) in growth chambers and glasshouses (Fiscus and Booker, 1995), or under balanced UV‐B fluences in the field, but at diverse locations with dissimilar environmental conditions (Rozema et al., 1997). A few studies have simultaneously compared responses of several species and/or their varieties to realistic UV‐B fluences at the same field location (Reed et al., 1992; Barnes et al., 1993; Correia et al., 1998), but none have included Southern hemisphere taxa. There are no comparative studies of species of ecological and economic importance for the Southern hemisphere. Clearly, there is a need to assess the relative sensitivity of Southern hemisphere species and varieties of different growth form and functional type to UV‐B supplements of realistic magnitude under similar environmental conditions, so that the impacts of the changing environment on agricultural production and natural ecosystems can be assessed.
MATERIALS AND METHODS
Species and seed sources
Seventeen tree, shrub and herb species of ecological and commercial importance in southern Africa were investigated. Trees included four forms (ecotypes) of the deciduous, sub‐tropical species Colophospermum mopane (Kirk ex Benth) Kirk ex J Leon alba, chota, leslie and ovifolia (seed source: University of the North, South Africa). C. mopane is a main source of winter fodder for South African game‐farms, bringing in about US$14 million annually, and of food for mopane worms, an industry that earns Botswana $8 million annually. It is also a source of medicines for traditional healers and is used extensively for fencing, rope, firewood and building materials (Madzibane and Potgieter, 1999). Another tree species, Virgilia oroboides (Bergius) T.M. Salter (seed source: Kirstenbosch Gardens, South Africa), an evergreen, temperate species, was also studied.
The shrubs studied were the evergreen temperate species Aspalathus linearis (Burm. f.) R. Dahlgren, Barleria obtusa Nees, Cliffortia ruscifolia L., Cyclopia genistoides (L.) Vent, Hermania saccilifera (Turcz.) K. Schum, Leuca dendron laureolum (Lam.) Fourc., Phylica pubescens Aiton., Podalyria calyptrata Willd. and Protea burchellii Stapf (seed source: Kirstenbosch Botanical Gardens, South Africa). Proteaceae (L. laureloum, P. burchelii) are of especial importance to the South African wild‐flower industry with annual export earnings of $13·8 million (Cunningham and Davis, 1997). A. linearis and C. genistoides are herbal beverages (Morton, 1983) with annual export earnings of $2 million (Cunningham and Davis, 1997).
The herbs studied were the annuals used in tropical agriculture, namely Glycine max (L.) Merr. ‘Prima’, Phaseolus vulgaris L. ‘PAN 159’ (seed source: University of Potchefstroom, South Africa), a Vigna subterranea L. landrace and Vigna unguiculata L. Walp. landrace Bengpilaa, both originating from Ghana (seed source: Nadowli District, Ghana), and the pasture annuals Lupinus luteus L. and Vicia atropurpurea Desf. (seed source: Kirstenbosch Botanical Gardens, South Africa) from more temperate climates.
UV‐treatments and growing conditions
Seeds of temperate‐climate species from winter rainfall regions were sown in May, while those of tropical‐climate species from summer rainfall regions were sown during December (Fig. 1A). Approximately ten seeds of each species were sown into mixtures of coarse sand and vermiculite (2 : 1, v/v) contained in 20 cm high × 20 cm diameter pots. Pots were irrigated daily with tap water to maintain a moist soil. Seedlings were thinned to two per pot 2 weeks after germination. Plants were provided with 66·6 mg K, 34·2 mg Ca, 20·7 mg Mg, 22·4 mg N and 24·8 mg P each week by adding 400 ml 50 % strength modified Hoagland’s solution (Hewitt, 1966) to each pot twice weekly.
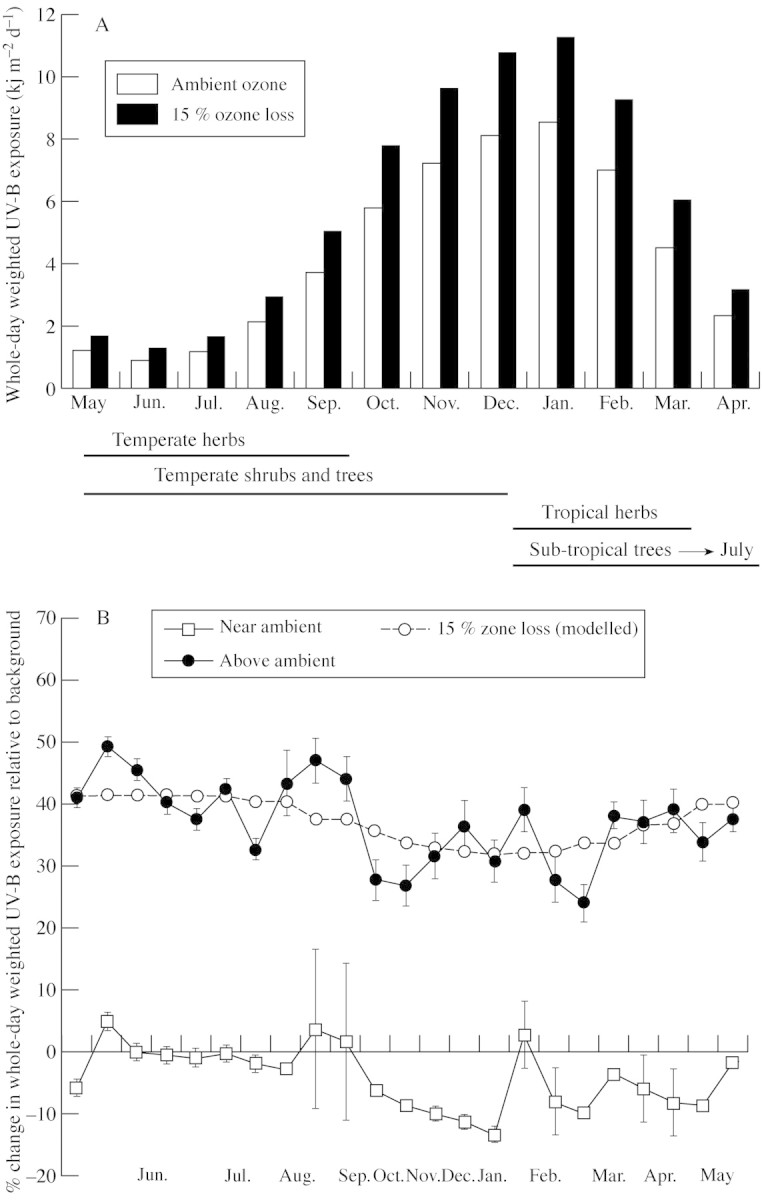
Fig. 1. A, Modelled average daily UV‐B exposures for ambient ozone and 15 % ozone depletion over experimental growing periods of herbs, shrubs and trees. B, Measured percentage changes in UV‐B flux relative to background beneath mylar‐filtered (ambient UV‐B) and cellulose‐diacetate‐filtered (enhanced UV‐B) lamps.
The experimental treatments were applied by eight separate banks of fluorescent sun lamps (Phillips TL/12 40W UV‐B, The Netherlands) located in an open area in the Kirstenbosch National Botanical Gardens, Cape Town, South Africa (36°56′S, 18°29′E). Four pots of each species were randomized under each bank of lamps. For near‐ambient UV‐B treatments, lamps in alternate banks were filtered (no transmission below 316 nm) with 0·12 mm thick Mylar‐D film (DuPont De Nemours, Wilmington, DE, USA). For above‐ambient UV‐B treatments, lamps in intervening banks were filtered (transmission down to 290 nm) with 0·075 mm thick cellulose acetate film (Courtaulds Chemicals, Derby, UK). All filters were replaced weekly to ensure uniformity of UV transmission. Artificial UV‐B radiation was supplied for 8 h per day, but supply was graduated, with two‐thirds of the total daily UV‐B supplement spread over a 4 h photoperiod centred on the solar noon (1300 h South African Standard Time). The remaining third was applied equally over the two 2 h early morning and late afternoon photoperiods. This was achieved by switching on fewer lamps in each bank during these photoperiods. Artificial UV‐B supplements were supplied mostly under clear‐sky conditions, as lamps were switched off during cloudy periods caused by the passage of intermittent cold fronts.
Spectral irradiances of filtered lamps were measured after sunset with a computer‐interfaced monochromator spectroradiometer (IL‐1700; International Light Inc., Newburyport, MA, USA), calibrated for absolute response and checked for wavelength alignment. Measured irradiances were weighted with a generalized plant response action spectrum (Caldwell, 1971), as formulated mathematically by Green et al. (1974), which was normalized at 300 nm. Weighted irradiances were integrated over the wavelength range 280–315 nm and expressed as a function of distance from the lamp source. Distances between cellulose acetate filtered lamps and the median height of plants in each bank were adjusted to increase UV‐B by an average of 37 % (seasonal range 41·3 % in winter to 31·8 % in summer) above modelled clear‐sky background flux (Fig. 1B). This increase is expected from a 15 % depletion in total column ozone above Cape Town according to a computerized (Musil and Bhagwandin, 1992) semi‐empirical model (Green, 1983). The experimentally simulated depletion of stratospheric ozone slightly exceeded the predicted 11 % for all seasons at Southern hemisphere mid‐latitudes (Madronich et al., 1995). Due to differences in plant heights, the range of supplemental UV‐B applied to plants under each bank was ±6·5 % of the mean for temperate climate species and ±4·6 % for tropical species. Lamps in the mylar‐filtered controls were fixed at the same distances above plants as the UV‐B treatments to provide similar UV‐A exposures in both (Newsham et al., 1996). Heights of lamps were adjusted regularly to accommodate median increases in plant height in each bank and seasonal variations in UV‐B exposure. Adjustments were checked with a UV‐B biometer sensor (Model 3D‐600; Solar Light Company, PA, USA), calibrated against the spectroradiometer for the generalized plant action spectrum, which regularly measured percentage changes in UV‐B at median plant heights beneath the lamps. Measured UV‐B exposures above background fluxes averaged –4·2 % (seasonal range –13·3–4·8 %) in the controls and 37·1 % (seasonal range 24·1–9·2 %) in the UV‐B treatments (Fig. 1B).
Pigments and metabolites
Concentrations of photosynthetic pigments, flavonoids and non‐structural carbohydrates were determined in leaves randomly collected at midday from apical portions of mature plants.
Photosynthetic pigments were extracted from fresh leaf samples ground at a low light intensity in 10 ml 100 % methanol at 2 °C. Absorbances of centrifuged extracts were measured with a spectrophotometer (Beckman DU 640; Beckman Instruments Inc., Fullerton, CA, USA) at specified wavelengths required for computation of chlorophyll a, chlorophyll b and total carotenoid concentrations from published formulae (Lichtenthaler, 1987). Leaf pellets remaining after centrifugation were dried at 60 °C in a forced draft oven and weighed. Photosynthetic pigment concentrations were expressed as µg mg–1 leaf dry mass.
Flavonoids (methanol extractable UV‐B absorbing compounds) were extracted from fresh leaf samples ground in 10 ml acidified methanol (79 : 20 : 1, v/v, methanol : water : HCl). Absorbances of centrifuged extracts were measured at 300 nm after appropriate dilution (Mireki and Teramura, 1984). Leaf pellets remaining after centrifugation were dried at 60 °C in a forced draft oven and weighed. Flavonoid concentrations were expressed as absorbance (Ab) at 300 nm g–1 leaf dry mass.
Total soluble sugars (sucrose, glucose and fructose) were extracted from fresh leaf samples. These were ground in two ×10 ml volumes of 80 % ethanol (80 : 20, v/v, ethanol : water), centrifuged and supernatants adjusted to 25 ml in volumetric flasks for spectrophotometric determination of total soluble sugars (Buysse and Merckx, 1993). Residues were dried at 60 °C and weighed. Starch concentrations were analysed by hydrolysing the residues in 5 ml 3·6 % HCl at 100 °C for 3 h, centrifuging and analysing the resultant sugars in the extracts (Buysse and Merckx, 1993). Soluble sugar and starch concentrations were expressed as µg mg–1 leaf dry mass. Non‐structural carbohydrate concentrations were calculated as the sum of total soluble sugars and starch.
Leaf, morphology, plant canopy area and dry mass
Herbs were harvested when they commenced flowering; tree and shrub species were harvested 8 months after sowing (Fig. 1A). Moribund and insect‐damaged individuals were excluded from the harvest. Visibly healthy plants were separated into leaf, branch and root fractions. The areas and shape indices (ratio of leaf perimeter to its area) of all leaves present on each plant were determined with an image analyser (Delta T Devices Ltd, Cambridge, UK). The plant fractions were dried at 60 °C and dry masses determined. Specific leaf area (SLA, cm2 g–1 leaf) was calculated as the ratio of leaf area to dry mass, and canopy area as the sum of all individual leaf areas per plant. Total plant dry mass was the sum of leaf, branch and root masses, and leaf area ratio (LAR, cm2 g–1 plant) was the ratio of canopy area to total plant dry mass.
Statistical analyses
All measurements were loge transformed to reduce inequality of variance in the raw data. Effects of UV‐B on all measured leaf biochemical and morphological properties, plant canopy area and dry mass were tested at the plant growth form and species levels. A REML (residual maximum likelihood) variance component analysis (Genstat, 1993), which efficiently estimates treatment effects in unbalanced experimental designs with more than one source of error, was used to test the fixed effects of UV‐B, plant growth form and UV‐B × plant growth form. Three models were investigated for the random effects of lamp banks, lamp banks × plant growth form, and lamp banks × plant growth form × species. The estimated variance components indicated the absence of any significant (P ≥ 0·05) effect of lamp bank or lamp bank × plant growth form (Table 1). Therefore, it was considered unnecessary to account for these effects in the REML analysis and only random effects due to plant growth form and plant growth form × species were used in the modelling process. All fixed effects were judged against the combined effect of these. UV‐B effects on species were tested with a single‐factor ANOVA where lamp banks comprised the treatment replicates. Correlation coefficients and a student’s t‐test tested statistical correspondence between measured changes in leaf biochemical and morphological properties, canopy area and dry mass of all species in response to UV‐B enhancement.
Table 1.
. Estimated variance components ± s.e. for random effects of lamp banks, plant growth form and species in the experimental design
| Random terms | Lamp banks | Lamp bank × plant growth form | Lamp bank × plant growth form × species | Units (plants) |
| Leaf pigments and metabolites | ||||
| Chlorophyll a | 0·00052 ± 0·00584 | 0·00001 ± 0·00981 | 0·17198 ± 0·01996 | 0·05683 ± 0·00464 |
| Chlorophyll b | 0·00013 ± 0·00680 | 0·00001 ± 0·01169 | 0·19585 ± 0·02391 | 0·08639 ± 0·00704 |
| Carotenoids | 0·00085 ± 0·00702 | 0·00001 ± 0·01170 | 0·21188 ± 0·02373 | 0·05355 ± 0·00437 |
| Flavonoids | 0·00100 ± 0·00628 | 0·00000 ± 0·01030 | 0·18584 ± 0·02088 | 0·04863 ± 0·00393 |
| Non‐structural carbohydrates | 0·00001 ± 0·01037 | 0·00001 ± 0·01801 | 0·32341 ± 0·03664 | 0·08894 ± 0·00729 |
| Leaf area, morphology and plant biomass | ||||
| SLA | 0·00030 ± 0·00327 | 0·00000 ± 0·00560 | 0·08470 ± 0·01099 | 0·03732 ± 0·00337 |
| Leaf shape index | 0·000000 ± 0·000670 | 0·000000 ± 0·001231 | 0·013907 ± 0·001969 | 0·001245 ± 0·000146 |
| Canopy area | 0·0151 ± 0·0565 | 0·0000 ± 0·0900 | 1·4842 ± 0·1704 | 0·1861 ± 0·0177 |
| Plant dry mass | 0·0307 ± 0·0444 | 0·0000 ± 0·0578 | 0·9341 ± 0·1091 | 0·1479 ± 0·0141 |
| LAR | 0·00001 ± 0·01032 | 0·00001 ± 0·01815 | 0·26591 ± 0·03476 | 0·09882 ± 0·00942 |
Variance components exceeding two s.e. significant at P < 0·05.
RESULTS AND DISCUSSION
Leaf pigments and metabolites
Wald χ2 statistics indicated significant (P < 0·05) differences in the response to UV‐B by different growth forms for all measured leaf biochemical properties, with the exception of non‐structural carbohydrate concentrations (Table 2). Trees displayed significantly (P < 0·05) altered leaf chlorophyll a and b, carotenoid and flavonoid concentrations under elevated UV‐B, but this was not apparent in shrubs or herbs (Table 2). The ostensibly greater responsiveness of trees to UV‐B enhancement may have been biased by the high summertime UV‐B fluxes to which four of the five tree taxa were exposed during particularly UV‐B sensitive early developmental stages (Teramura and Sullivan, 1987). However, the arable tropical herbs were also exposed to high summertime UV‐B fluxes during early developmental stages (Fig. 1A), yet their responses to UV‐B enhancement contrasted with those of trees both in magnitude and direction (Table 3).
Table 2.
REML (residual maximum likelihood) variance component analysis statistics
| Predicted means (loge) | Wald tests for fixed effects | |||||||||||
| Herbs | Shrubs | Trees | Plant growth form | UV‐B | Plant growth form × UV‐B | |||||||
| Plant parameter | Ambient UV‐B | Enhanced UV‐B | Ambient UV‐B | Enhanced UV‐B | Ambient UV‐B | Enhanced UV‐B | Wald χ2 statistic | d.f. | Wald χ2 statistic | d.f. | Wald χ2 statistic | d.f. |
| Leaf pigments and metabolites | ||||||||||||
| Chlorophyll a | 2·286a | 2·233a | 1·005b | 1·040b | 1·222b | 1·379c | 34·9*** | 2 | 0·9 | 1 | 10·6** | 2 |
| Chlorophyll b | 0·696a | 0·622a | –0·686b | –0·652b | –0·238b | –0·109c | 35·7*** | 2 | 0·1 | 1 | 6·3* | 2 |
| Carotenoids | 1·213a | 1·161a | 0·022b | 0·015b | –0·020b | 0·123c | 27·3*** | 2 | 0·0 | 1 | 9·7** | 2 |
| Flavonoids | 4·760a | 4·830a | 5·203a | 5·180a | 4·164b | 4·386c | 15·0*** | 2 | 5·5* | 1 | 17·2*** | 2 |
| Non–structural carbohydrates | 5·687a | 5·664a | 5·470a | 5·506a | 4·592b | 4·528b | 12·2** | 2 | 0·0 | 1 | 2·0 | 2 |
| Leaf morphology, plant canopy area and dry mass | ||||||||||||
| SLA | 5·434a | 5·395a | 4·362b | 4·378b | 4·846c | 4·876c | 48·9*** | 2 | 0·1 | 1 | 2·7 | 2 |
| Leaf shape index | 0·195a | 0·203a | 0·345a | 0·339a | 0·164a | 0·172a | 5·0 | 2 | 0·3 | 1 | 1·9 | 2 |
| Canopy area | 6·399a | 6·153a | 5·466a | 5·370a | 4·913b | 4·761b | 4·2 | 2 | 11·0*** | 1 | 1·9 | 2 |
| Plant dry mass | 2·06a | 1·854a | 1·954a | 1·850a | 1·491a | 1·448a | 0·8 | 2 | 8·1** | 1 | 1·9 | 2 |
| LAR | 4·296a | 4·283a | 3·512b | 3·520b | 3·424b | 3·322b | 10·1** | 2 | 0·5 | 1 | 1·9 | 2 |
Differences between predicted means with different letters exceeding two s.e. and significant at P < 0·05.
Significant effects at * P < 0·05, ** P < 0·01, *** P < 0·001 presented in bold type.
Table 3.
Effects of elevated UV‐B radiation on leaf pigments and metabolites of different species
| Chlorophyll a (µg mg–1) | Chlorophyll b (µg mg–1) | Carotenoids (µg mg–1) | Flavonoids Abs (300 nm) g–1 | Non‐structural carbohydrates (µg mg–1) | |||||||||||
| Plant species/form | Ambient UV‐B | Enhanced UV‐B | F‐ratios | Ambient UV‐B | Enhanced UV‐B | F‐ratios | Ambient UV‐B | Enhanced UV‐B | F‐ratios | Ambient UV‐B | Enhanced UV‐B | F‐ratios | Ambient UV‐B | Enhanced UV‐B | F‐ratios |
| Trees | |||||||||||||||
| Colophospermum mopane | |||||||||||||||
| f. alba | 2·459 | 3·492 | 13·3** | 0·555 | 0·753 | 9·9** | 0·660 | 0·963 | 14·8** | 57·1 | 89·1 | 13·4** | 112·2 | 75·8 | 21·7*** |
| f. chota | 2·464 | 2·609 | 0·01 | 0·592 | 0·600 | 0·00 | 0·649 | 0·745 | 0·54 | 50·8 | 99·5 | 40·1*** | 70·2 | 73·2 | 0·03 |
| f. leslie | 2·673 | 3·996 | 40·5*** | 0·671 | 0·850 | 6·6* | 0·784 | 1·112 | 24·3*** | 59·0 | 68·5 | 2·10 | 86·6 | 65·8 | 27·8*** |
| f. ovifolia | 3·318 | 3·797 | 4·8* | 0·690 | 0·811 | 8·9** | 0·969 | 1·043 | 1·68 | 59·5 | 82·6 | 11·4** | 91·3 | 95·7 | 0·02 |
| Virgilia oroboides | 8·717 | 9·055 | 0·13 | 2·115 | 2·211 | 0·09 | 2·809 | 2·849 | 0·00 | 94·2 | 96·0 | 0·04 | 198·1 | 221·0 | 0·42 |
| Shrubs | |||||||||||||||
| Aspalathus linearis | 3·059 | 2·359 | 1·51 | 0·679 | 0·511 | 1·31 | 0·810 | 0·727 | 0·41 | 118·2 | 106·1 | 0·88 | 192·4 | 158·8 | 1·74 |
| Barleria obtusa | 3·752 | 3·553 | 0·29 | 0·766 | 0·471 | 17·1*** | 1·628 | 1·700 | 0·38 | 386·4 | 374·3 | 0·00 | 298·3 | 305·6 | 0·11 |
| Cliffortia ruscifolia | 2·862 | 2·745 | 1·00 | 0·599 | 0·560 | 1·65 | 0·881 | 0·856 | 0·08 | 165·6 | 158·0 | 0·26 | 239·4 | 244·9 | 0·00 |
| Cyclopia genistoides | 5·709 | 4·882 | 1·84 | 1·221 | 1·028 | 0·97 | 2·107 | 1·854 | 1·50 | 205·1 | 189·8 | 1·49 | 548·8 | 508·6 | 1·21 |
| Hermannia saccilifera | 4·242 | 4·744 | 2·51 | 0·440 | 0·461 | 0·00 | 1·783 | 1·918 | 0·95 | 403·6 | 311·8 | 9·7** | 292·9 | 342·2 | 6·5* |
| Leucadendron laureolum | 1·673 | 1·971 | 20·6*** | 0·216 | 0·436 | 21·9*** | 0·697 | 0·683 | 0·19 | 148·5 | 149·8 | 0·00 | 191·5 | 228·1 | 8·5* |
| Phylica pubescens | 1·694 | 2·154 | 19·8*** | 0·328 | 0·429 | 4·5* | 0·577 | 0·686 | 14·4*** | 133·9 | 134·0 | 0·01 | 139·6 | 147·5 | 1·21 |
| Podalyria calyptrata | 3·161 | 3·204 | 0·00 | 0·835 | 0·846 | 0·03 | 1·021 | 1·048 | 0·01 | 69·0 | 73·5 | 0·50 | 272·3 | 281·5 | 0·25 |
| Protea burchellii | 1·695 | 1·968 | 0·03 | 0·386 | 0·379 | 0·45 | 0·847 | 0·818 | 1·09 | 346·4 | 399·1 | 1·73 | 194·1 | 222·1 | 6·2* |
| Herbs | |||||||||||||||
| Glycine max | 10·091 | 9·823 | 0·53 | 2·045 | 2·105 | 0·34 | 3·366 | 3·176 | 1·67 | 145·0 | 164·7 | 10·3** | 518·0 | 506·9 | 0·41 |
| Lupinus luteus | 11·311 | 11·241 | 0·13 | 2·087 | 2·037 | 0·17 | 4·442 | 4·462 | 0·00 | 128·8 | 127·7 | 0·16 | 125·5 | 122·7 | 0·00 |
| Phaseolus vulgaris | 7·609 | 6·440 | 1·01 | 1·741 | 1·307 | 3·10 | 2·463 | 2·123 | 0·80 | 123·2 | 134·9 | 1·20 | 596·9 | 659·4 | 2·25 |
| Vicia atropurpurea | 16·547 | 16·603 | 0·00 | 3·799 | 3·672 | 0·40 | 5·561 | 5·659 | 0·11 | 80·2 | 91·6 | 2·10 | 104·1 | 97·4 | 0·38 |
| Vigna subterranea | 8·134 | 8·488 | 0·92 | 1·458 | 1·589 | 1·23 | 3·027 | 3·091 | 0·25 | 149·8 | 145·5 | 0·58 | 349·8 | 286·8 | 2·92 |
| Vigna unguiculata | 8·682 | 7·385 | 5·7* | 1·972 | 1·549 | 8·3** | 2·607 | 2·290 | 3·76 | 102·1 | 117·8 | 2·09 | 616·1 | 725·8 | 1·42 |
Significant contrasts at *P < 0·05, **P < 0·01, ***P < 0·001 presented in bold type.
Only five species displayed significantly (P < 0·05) altered leaf biochemical properties under elevated UV‐B, but there was considerable variability both between and within these species as to which biochemical properties were altered and the magnitude and direction of change (Table 3).
Concentrations of chlorophyll a and b were significantly (P < 0·05) altered in leaves of five species (Table 3). In the Colophospermum mopane tree ecotypes alba, leslie and ovifolia, and in the shrubs Leucadendron laureolum and Phylica pubescens, they were increased—an uncommon response to UV‐B enhancement. Increases have been reported previously, but usually when chlorophyll concentrations were expressed on a leaf dry mass basis (Alenius et al., 1995; Smith et al., 2000). This would occur if leaf density increased (decreased SLA). However, changes in SLA of species under elevated UV‐B were not significantly correlated with corresponding changes in their leaf chlorophyll content. The decreased chlorophyll concentration, especially that of chlorophyll b, measured in the shrub Barleria obtusa and the herb Vigna unguiculata is a more common symptom of UV‐B radiation stress. This is attributed to increased photo‐degradation of chlorophylls (Strid and Porra, 1992) and lower rates of chlorophyll synthesis resulting from reduced expression of genes encoding chlorophyll‐binding proteins (Strid et al., 1994).
Concentrations of carotenoids were significantly (P < 0·01) increased in leaves of two species, the C. mopane tree ecotypes alba and leslie and in the shrub P. pubescens (Table 3). These may represent a biochemical response to alleviate UV‐B stress since carotenoids function in the photo‐protection of photosynthetic systems by dissipating excess excitation energy through the xanthophyll cycle (Demmig‐Adams and Adams, 1992).
Flavonoid concentrations were significantly (P < 0·01) altered in leaves of three species (Table 3). The C. mopane tree ecotypes alba, chota and ovifolia and the herb Glycine max had increased concentrations, a consistently reported response to UV‐B enhancement (Flint et al., 1985; Barnes et al., 1987). The shrub Hermania saccilifera had a decreased flavonoid concentration, which did not correlate with reductions in its leaf density, i.e. increase in SLA (Table 4). There was also no evidence to support suggestions that species with naturally high flavonoid contents in their leaves are more resilient to UV‐B enhancements (Sullivan et al., 1992; Ziska et al., 1992). Absolute flavo noid concentrations of species in near‐ambient UV‐B treatments were not significantly correlated with corresponding changes in their total dry mass or canopy area under enhanced UV‐B. Furthermore, the data did concur with proposals that species that synthesize additional flavonoids in response to UV‐B exposure are better protected against UV‐B damage (Murali and Teramura, 1986), and vice versa (Lois and Buchanan, 1994). No significant correlations were found between the amounts of flavonoids accumulated by species under enhanced UV‐B and corresponding changes in their total dry mass and canopy area. Bornman and Vogelmann (1991) concluded that it is the cellular location of UV‐B‐screening phenylpropanoid pigments, rather than the total amounts present in leaves, that is of importance in UV‐B protection.
Table 4.
Effects of elevated UV‐B radiation on leaf area, morphology, plant canopy area and dry mass of different species
| SLA | Leaf shape index | Plant canopy area (cm2) | Plant dry mass (g) | LAR | |||||||||||
| Plant species/form | Ambient UV‐B | Enhanced UV‐B | F‐ratios | Ambient UV‐B | Enhanced UV‐B | F‐ratios | Ambient UV‐B | Enhanced UV‐B | F‐ratios | Ambient UV‐B | Enhanced UV‐B | F‐ratios | Ambient UV‐B | Enhanced UV‐B | F‐ratios |
| Trees | |||||||||||||||
| Colophospermum mopane | |||||||||||||||
| f. alba | 104·3 | 113·8 | 2·14 | 1·183 | 1·177 | 0·52 | 66·4 | 81·6 | 1·45 | 3·192 | 4·341 | 8·7** | 20·6 | 17·1 | 0·27 |
| f. chota | 106·3 | 122·1 | 9·7** | 1·169 | 1·199 | 12·9** | 97·2 | 52·3 | 10·4** | 3·647 | 2·234 | 11·2** | 26·6 | 22·4 | 2·82 |
| f. leslie | 126·1 | 115·5 | 1·92 | 1·178 | 1·199 | 3·19 | 96·6 | 57·9 | 13·7** | 4·000 | 3·814 | 0·02 | 26·1 | 13·2 | 9·4** |
| f. ovifolia | 143·6 | 126·5 | 4·6* | 1·182 | 1·180 | 0·03 | 81·9 | 63·0 | 4·3 | 2·088 | 1·849 | 0·63 | 50·5 | 41·7 | 0·54 |
| Virgilia oroboides | 179·8 | 189·6 | 1·73 | – | – | – | 1779·6 | 1720·4 | 0·00 | 30·359 | 28·317 | 0·12 | 58·6 | 61·9 | 0·86 |
| Shrubs | |||||||||||||||
| Aspalathus linearis | 55·6 | 54·7 | 0·19 | – | – | – | 146·5 | 96·8 | 3·33 | 7·680 | 6·617 | 0·47 | 19·1 | 15·0 | 7·0* |
| Barleria obtusa | 83·1 | 96·2 | 2·29 | 1·147 | 1·136 | 4·5* | 184·7 | 200·5 | 0·91 | 6·813 | 5·863 | 8·2** | 27·1 | 34·2 | 7·2* |
| Cliffortia ruscifolia | 48·3 | 45·5 | 1·02 | 1·486 | 1·456 | 2·88 | 466·1 | 472·4 | 0·01 | 16·408 | 17·175 | 0·56 | 28·8 | 27·4 | 0·48 |
| Cyclopia genistoides | 108·5 | 113·0 | 0·42 | – | – | – | 1107·4 | 923·5 | 2·92 | 29·731 | 23·811 | 5·7* | 37·3 | 38·8 | 0·04 |
| Hermannia saccilifera | 70·2 | 62·9 | 1·45 | 1·169 | 1·186 | 3·16 | 190·2 | 194·5 | 0·01 | 7·267 | 7·492 | 0·06 | 26·8 | 26·1 | 0·02 |
| Leucadendron laureolum | 105·6 | 118·9 | 0·40 | 1·889 | 1·915 | 0·02 | 248·4 | 354·0 | 0·87 | 3·735 | 6·070 | 0·49 | 71·4 | 72·1 | 0·04 |
| Phylica pubescens | 89·4 | 91·5 | 0·01 | 1·511 | 1·496 | 0·16 | 108·1 | 102·1 | 0·12 | 2·251 | 2·094 | 0·41 | 47·1 | 51·0 | 0·26 |
| Podalyria calyptrata | 98·1 | 100·8 | 0·24 | – | – | – | 1040·9 | 955·3 | 1·10 | 29·580 | 27·220 | 0·92 | 36·1 | 34·9 | 0·21 |
| Protea burchellii | 92·0 | 79·7 | 0·24 | – | – | – | 52·4 | 42·1 | 0·50 | 1·484 | 1·183 | 0·54 | 40·0 | 40·7 | 0·17 |
| Herbs | |||||||||||||||
| Glycine max | 195·9 | 198·1 | 0·30 | 1·136 | 1·163 | 19·7*** | 1086·3 | 917·4 | 1·37 | 14·637 | 12·999 | 5·4* | 74·6 | 71·9 | 0·06 |
| Lupinus luteus | 251·4 | 241·6 | 3·97 | – | – | – | 591·9 | 538·5 | 0·97 | 9·037 | 8·368 | 0·60 | 65·6 | 64·0 | 1·51 |
| Phaseolus vulgaris | 280·7 | 288·1 | 1·03 | 1·214 | 1·220 | 0·22 | 168·9 | 191·9 | 0·12 | 4·363 | 7·138 | 2·12 | 34·7 | 25·4 | 0·00 |
| Vicia atropurpurea | 349·4 | 345·0 | 0·12 | – | – | – | 3772·0 | 3803·7 | 0·05 | 18·003 | 17·432 | 0·00 | 206·8 | 218·3 | 0·24 |
| Vigna subterranea | 198·8 | 190·6 | 0·00 | 1·396 | 1·367 | 1·29 | 261·4 | 401·2 | 0·69 | 2·068 | 3·327 | 0·99 | 127·7 | 123·4 | 0·58 |
| Vigna unguiculata | 166·2 | 142·3 | 2·44 | 1·137 | 1·162 | 16·3*** | 512·7 | 250·1 | 35·5*** | 9·629 | 5·080 | 24·9*** | 54·4 | 55·7 | 0·00 |
Significant contrasts at *P < 0·05, **P < 0·01, ***P < 0·001 presented in bold type.
Non‐structural carbohydrate concentrations were significantly (P < 0·05) altered in leaves of four species (Table 3). The C. mopane tree ecotypes alba and leslie had decreased concentrations, which did not correlate with reductions in their leaf density, i.e. increase in SLA (Table 4). The shrubs L. laureolum, P. pubescens and Protea burchellii had increased non‐structural carbohydrate concentrations. Such increases have also been reported in UV‐B‐irradiated leaves of pea, corn and a desert annual (Santos et al., 1993; He et al., 1994; Musil et al., 1999). They were attributed to mitochondrial damage resulting from decreased respiratory consumption of substrate favouring starch accumulation (Santos et al., 1993; He et al., 1994), since the absence of any UV‐B effect on phosphoenolpyruvate or Ribulose‐1,5,‐bisphosphate carboxylase/oxygenase (Rubisco) activity indicated that starch accumulation was not due to photosynthetic stimulation (Santos et al., 1993). Current opinion is that cellular accumulation of starch can inhibit photosynthesis through several routes, including physical disruption of chloroplast function, feedback inhibition of photosynthesis due to reduced Rubisco activity and inorganic phosphate limitation (Cave et al., 1981; Stitt, 1991), and this may diminish biomass production. However, no significant correlations were found between the amounts of non‐structural carbohydrates accumulated by species under enhanced UV‐B and corresponding changes in their total dry mass and canopy area.
Plant canopy area and dry mass
Wald χ2 statistics indicated a significant (P < 0·01) main effect of UV‐B on plant canopy area and dry mass that was independent of plant growth form, and the absence of a significant (P ≥ 0·05) UV‐B × plant growth form interaction (Table 2). These findings are inconsistent with reported differences in susceptibility to UV‐B damage among different plant growth forms related to the effectiveness of their foliage in screening out UV‐B (Day et al., 1992, 1994). Epidermal transmittance and depth of penetration of UV‐B into foliage was smallest in evergreen trees and shrubs, intermediate in woody dicotyledons and grasses, and greatest in herbaceous dicotyledons (Day, 1993).
The small effects of UV‐B enhancement in the different plant growth forms were also apparent in the comparison of species. A significantly increased dry mass under elevated UV‐B was observed only in the C. mopane tree ecotype alba. One other C. mopane tree ecotype, chota, as well as the shrubs B. obtusa and Cyclopia genistoides and the herbs G. max and V. unguiculata had decreased dry masses (Table 4). Reduced canopy areas were evident in C. mopane tree ecotypes chota and leslie and in the herb V. unguiculata. Canopy area reductions in these species (40 % and greater) were more than double those of other taxa, and this also applied to reductions in their dry mass, showing that they are especially sensitive to UV‐B. Other studies of Southern hemisphere trees, shrubs and herbs have also reported varied UV‐B effects on biomass accumulation and photosynthetic capacity in different species. Among trees, decreased photosynthesis accompanied by a tendency towards reduced biomass production occurred in Acacia tortilis saplings exposed for 190 d to a 25 % increase in background UV‐B radiation in an African subtropical‐climate savanna (Ernst et al., 1997). However, photosynthesis and biomass accumulation were not altered in saplings of the closely related A. karroo following similar (30 week) exposure to a 38 % increase in background UV‐B radiation for an African temperate‐climate savanna (Wand et al., 1996). In contrast, two shade‐tolerant New Zealand tree species suffered a 20 % decline in photochemical efficiency and one shade‐intolerant species a 10 % decline (Hunt et al., 1996). However, the UV‐B applied (16·8 kJ m–2 d–1) was about twice that of clear‐sky summer days in New Zealand, and the intensity at which it was applied was at least six‐times that occurring at the solar noon. Among evergreen shrubs, only one of three sclerophyllous Ericaceae species from a Mediterranean climate region in South Africa displayed photosynthetic inhibition following exposure to 21 and 46 % increases in UV‐B radiation. This inhibition was due to an indirect UV‐B effect on stomatal function only (Musil and Wand, 1993). These findings also concur with observations on evergreen shrubs from Mediterranean climates in the Northern hemisphere. Growth was inhibited under natural precipitation in only one of six European evergreen shrub species exposed to a 35 % increase in background UV‐B radiation, with additional watering abolishing the growth inhibition caused by the increased UV‐B (Manetas, 1999). As regards herbaceous dicotyledons, a recent New Zealand study demonstrated reductions in whole plant biomass in seven of 19 vegetable crop species and varieties examined. However, these were in response to UV‐B (15·8 kJ m–2 d–1) nearly twice that on a clear summer day in New Zealand, with controls (0·05 kJ m–2 d–1) having almost no UV‐B (Smith et al., 2000).
Leaf morphology
Wald χ2 statistics indicated no significant (P ≥ 0·05) main effects of UV‐B, growth form, or UV‐B × growth form interactions on SLA and leaf shape (Table 2). SLA was significantly (P < 0·05) altered in the C. mopane tree ecotypes chota and ovifolia, but was affected differently in each ecotype (Table 4). Increased leaf thickness enhances internal light scattering, thus reducing the harmful effects of incident UV‐B on sensitive photosynthetic tissues (Bornman and Vogelmann, 1991). Noteworthy was that the C. mopane tree ecotype ovifolia, whose SLA decreased (increased leaf thickness) in response to UV‐B enhancement, suffered no significant reductions in canopy area and dry mass. Conversely, the C. mopane tree ecotype chota, with an increased SLA (decreased leaf thickness) in response to UV‐B, suffered significant reductions in both canopy area and dry mass. Indeed, plants with low SLA (thick leaves) and the capacity to reduce SLA (increase leaf thickness) in response to UV‐B enhancement are considered better protected against UV‐B damage (Murali and Teramura, 1985). However, no statistically significant correlations were found between the absolute SLA of species in near‐ambient UV‐B treatments, nor between the magnitude of change in SLA in species under enhanced UV‐B, and corresponding changes in their total dry mass and canopy area.
Alterations in leaf shape modify the area available for energy capture and may assist in reducing the harmful effects of incident UV‐B (Jansen et al., 1998). Significantly larger leaf shape indices, indicating more elongated leaves, were observed in the C. mopane tree ecotype chota and the herbs G. max and V. unguiculata (Table 4). Conversely, smaller leaf shape indices, indicating broader leaves, were observed in the shrub B. obtusa. Alterations in leaf shape represent one of a suite of photomorphogenic responses unrelated to changes in photosynthetic carbon assimilation and biomass production (Barnes et al., 1996) that are especially sensitive to changes in wavelength most affected by stratospheric ozone reduction (Ensminger, 1993). Current opinion is that such UV‐B‐induced photomorphogenic responses may change canopy structure, light interception, stand photosynthesis and thus competition between species (Barnes et al., 1996). Indeed, altered leaf shapes of species under elevated UV‐B were significantly correlated with corresponding changes in their canopy area (r2 = 0·2293, t1113 = 2·50, P ≥ 0·01) and dry mass (r2 = 0·2662, t1112 = 2·92, P ≥ 0·01), supporting the importance of UV‐B‐induced photomorphogenesis in indirectly altering the performance of species.
In conclusion, this analysis of 17 species, including ecotypes, of native southern African plants and crops grown in the region showed uncommon (<30 % of species affected) responses to realistic UV‐B enhancements, and variability in response similar to that between other species from, and grown in, different geographical areas. There was no pattern of response to UV‐B related to growth form. Neither was there any consistent pattern of change in species’ growth relating to altered leaf thickness and biochemical composition with increased UV‐B. These findings indicate that generalizations on plant sensitivity to UV‐B based on growth form and functional type could be misleading, and that the great majority of economically important species of the region are likely to be insensitive to future UV‐B increases. Notable exceptions include Colophospermum mopane tree ecotypes chota and leslie and the arable annual Vigna unguiculata, both of which are traditional sources of livelihood to rural African populations and of importance to African industry and agriculture.
ACKNOWLEDGEMENTS
We thank Ms J. Arnolds for assistance with analyses of plant pigments and metabolites, and Mr S. Snyders for maintenance of lamp systems and plants. The study was partly funded by the National Botanical Institute and by grants from the National Research Foundation and Deutscher Akademischer Austausch Dienst.
Supplementary Material
Received: 11 February 2002; Returned for revision: 4 March 2002; Accepted: 10 April 2002
References
- AleniusCM, Vogelmann TC, Bornman JF.1995. A three‐dimensional representation of the relationship between penetration of u.v.‐B radiation and u.v.‐screening pigments in leaves of Brassica napus New Phytolologist 131: 297–302. [Google Scholar]
- BarnesPW, Ballare CL, Caldwell MM.1996. Photomorphogenic effects of UV‐B radiation on plants: consequences for light competition Journal of Plant Physiology 148: 15–20. [Google Scholar]
- BarnesPW, Flint SD, Caldwell MM.1987. Photosynthesis damage and protective pigments in plants from a latitudinal arctic/alpine gradient exposed to supplemental UV‐B radiation in the field. Arctic Alpine Research 19: 21–27. [Google Scholar]
- BarnesPW, Maggard S, Holman SR, Vergara BS.1993. Intraspecific variation in sensitivity to UV‐B radiation in rice. Crop Science 33: 1041–1046. [Google Scholar]
- BornmanJF, Vogelmann TC.1991. Effect of UV‐B radiation on leaf optical properties measured with fibre optics. Journal of Experi mental Botany 42: 547–554. [Google Scholar]
- BuysseJ, Merckx R.1993. An improved colorimetric method to quantify sugar content of plant tissue. Journal of Experimental Botany 44: 1627–1629. [Google Scholar]
- CaldwellMM.1971. Solar ultraviolet radiation and the growth and development of higher plants. In: Giese AC, ed. Photophysiology, Vol. 6. New York: Academic Press, 131–177. [Google Scholar]
- CaldwellMM, Flint SD, Searles PS.1994. Spectral balance and UV‐B sensitivity of soybean: a field experiment. Plant Cell and Environment 17: 267–276. [Google Scholar]
- CaldwellMM, Teramura AH, Tevini M, Bornmann LO, Kulandaivelu G.1995. Effects of solar ultraviolet radiation on terrestrial plants. Ambio 24: 166–173. [Google Scholar]
- CaveG, Tolley LC, Strain BR.1981. Effect of carbon dioxide enrichment on chlorophyll content, starch content and starch grain structure in Trifolium subterraneum leaves. Physiologia Plantarum 51: 171–174. [Google Scholar]
- CorreiaCM, Areal ELV, Torres‐Pereira MS, Torres‐Pereira JMG.1998. Intraspecific variation in sensitivity to ultraviolet‐B radiation in maize grown under field conditions. I. Growth and morphological aspects. Field Crops Research 59: 81–89. [Google Scholar]
- CorreiaCM, Coutinho JF, Bjorn LO, Torres‐Pereira JMG.2000. Ultraviolet‐B radiation and nitrogen effects on growth and yield of maize under Mediterranean field conditions. European Journal of Agronomy 12: 117–125. [Google Scholar]
- CrutzenPJ.1992. Ultraviolet on the increase. Nature 356: 104–105. [Google Scholar]
- CunninghamAB, Davis GW.1997. Human use of plants. In: Cowling RM, Richardson DM, Pierce SM, eds. Vegetation of Southern Africa Cambridge: Cambridge University Press, 474–506. [Google Scholar]
- DawarS, Vani T, Singhal GS.1998. Stimulation of antioxidant enzymes and lipid peroxidation by UV‐B irradiation in the thylakoid membranes of wheat. Biologia Plantarum 41: 65–73. [Google Scholar]
- DayTA.1993. Relating UV‐B radiation screening effectiveness of foliage to absorbing‐compound concentration and anatomical characteristics in a diverse group of plants. Oecologia 95: 542–550. [DOI] [PubMed] [Google Scholar]
- DayTA, Howells BW, Rice WJ.1994. Ultraviolet absorption and epidermal‐transmittance spectra in foliage. Physiologia Plantarum 92: 207–218. [Google Scholar]
- DayTA, Vogelmann TC, DeLucia EH.1992. Are some plant life forms more effective at screening ultraviolet‐B radiation? Oecologia 92: 513–519. [DOI] [PubMed] [Google Scholar]
- Demmig‐AdamsB, Adams WW. III.1992. Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology 48: 609–639. [Google Scholar]
- EichhornM, Dohler G, Austen H.1993. Impact of UV‐B radiation on photosynthetic electron transport of Wolffia arrhiza (L.) Wimm. Photosynthetica 39: 613–618. [Google Scholar]
- EnsmingerPA.1993. Control of development in plants and fungi by far‐UV radiation. Physiologia Plantarum 88: 501–508. [Google Scholar]
- ErnstWHO, van de Staaij JWM, Nelissen HJM.1997. Reaction of savanna plants from Botswana on UV‐B radiation. Plant Ecology 128: 162–170. [Google Scholar]
- FiscusEL, Booker FL.1995. Is increased UV‐B a threat to crop photosynthesis and productivity? Photosynthesis Research 43: 81–92. [DOI] [PubMed] [Google Scholar]
- FlintSD, Jordan PW, Caldwell MM.1985. Plant protective response to enhanced UV‐B radiation under field conditions: leaf optical properties and photosynthesis. Photochemistry and Photobiology 41,95–99.4059360 [Google Scholar]
- Genstat.1993. Release 3 reference manual. Committee of the Statistical Department, Rothamsted Experimental Station. Oxford: Oxford Scientific Publications, Clarendon Press. [Google Scholar]
- GreenAES.1983. The penetration of ultraviolet radiation to the ground. Physiologia Plantarum 58: 351–359. [Google Scholar]
- GreenAES, Sawada T, Shettle EP.1974. The middle ultraviolet reaching the ground. Photochemistry and Photobiology 19: 251–259. [Google Scholar]
- Gwynn‐JonesD, Lee J, Johanson U, Phoenix G, Callaghan T, Sonesson M.1999. The responses of plant functional types to enhanced UV‐B radiation. In: Rozema J, ed. Stratospheric ozone depletion: the effects of enhanced UV‐B radiation on terrestrial ecosystems Leiden, The Netherlands: Backhuys Publishers, 173–185. [Google Scholar]
- HeJ, Huang L‐K, Whitecross MI.1994. Chloroplast ultrastructure changes in Pisum sativum associated with supplementary ultraviolet (UV‐B) radiation. Plant Cell and Environment 17: 771–775. [Google Scholar]
- HewittEJ.1966. Sand and water culture methods used in the study of plant nutrition, 2nd revised edition. Commonwealth Bureau of Horticultural and Plantation Crops, East Malling. Technical Communication No. 22. Farnham Royal, UK: Commonwealth Agricultural Bureau. [Google Scholar]
- HoqueE, Remus G.1999. Natural UV‐screening mechanisms of Norway spruce (Picea abies L. Karst) needles. Photochemistry and Photo biology 69: 177–192. [DOI] [PubMed] [Google Scholar]
- HuntJE, Neil DL.1998. Nitrogen status affects UV‐B sensitivity of cucumber. Australian Journal of Plant Physiology 25: 79–86. [Google Scholar]
- HuntJE, Kelliher FM, McNeil DL.1998. Response in chlorophyll a fluorescence of six New Zealand tree species to a step‐wise increase in ultraviolet‐B irradiance. New Zealand Journal of Botany 34: 401–410. [Google Scholar]
- JansenMAK, Gaba V, Greenberg BM.1998. Higher plants and UV‐B radiation: balancing damage, repair and acclimation. Trends in Plant Science 3: 131–135. [Google Scholar]
- LaaksoK, Huttunen S.1998. Effects of ultraviolet‐B radiation on conifers: a review. Environmental Pollution 99: 319–328. [DOI] [PubMed] [Google Scholar]
- LiJ, Ou‐Lee TM, Raba R, Amundson RG, Last RL.1993. Arabidopsis flavonoid mutants are hypersensitive to UV‐B irradiation. Plant Cell 5: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LichtenthalerHK.1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 148: 350–382. [Google Scholar]
- LoisR, Buchanan BB.1994. Severe sensitivity to ultraviolet radiation in an Arabidopsis mutant deficient in flavonoid accumulation. II. Mechanisms of UV‐resistance in Arabidopsis Planta 194: 504–509. [Google Scholar]
- McKenzieRL, Conner B, Bodeker GE.1999. Increased summertime UV radiation in New Zealand in response to ozone loss. Science 285: 1709–1711. [DOI] [PubMed] [Google Scholar]
- MadronichS, de Gruijl FR.1994. Stratospheric ozone depletion between 1979 and 1992. Implications for biologically active ultraviolet‐B radiation and non‐melanoma skin cancer incidence. Photochemistry and Photobiology 59: 541–546. [DOI] [PubMed] [Google Scholar]
- MadronichS, McKenzie RL, Caldwell MM, Björn L.O.1995. Changes in ultraviolet radiation reaching the earth’s surface. Ambio 24: 143–152. [DOI] [PubMed] [Google Scholar]
- MadzibaneJ, Potgieter MJ.1999. Uses of Colophospermum mopane (Leguminosae: Caesalpinioideae) by the Vhavenda. South African Journal of Botany 65: 440–443. [Google Scholar]
- ManetasY.1999. Is enhanced UV‐B radiation really damaging for plants? Some case studies with European Mediterranean species. In: Rozema J, ed. Stratospheric ozone depletion: the effects of enhanced UV‐B radiation on terrestrial ecosystems Leiden, The Netherlands: Backhuys Publishers, 251–263. [Google Scholar]
- MarkU, Tevini M.1997. Effects of solar ultraviolet‐B radiation, temperature and CO2 on growth and physiology of sunflower and maize seedlings. Plant Ecology 128: 224–234. [Google Scholar]
- MiddletonEM, Teramura AH.1994. Understanding photosynthesis, pigment and growth responses induced by UV‐B and UV‐A irradiances. Photochemistry and Photobiology 60: 38–45. [Google Scholar]
- MirekiRM, Teramura AH.1984. Effects of UV‐B irradiance on soybean. V. the dependence of plant sensitivity on the photosynthetic photon flux density during and after leaf expansion. Plant Physiology 74: 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MortonJF.1983. Rooibos tea, Aspalathus linearis, a caffeine‐less low tannin beverage. Economic Botany 37: 164–173. [Google Scholar]
- MuraliNS, Teramura AH.1985. Effects of UV‐B irradiance on soybean. VI. Influence of phosphorus nutrition on growth and flavonoid content. Physiologia Plantarum 63: 413–416. [Google Scholar]
- MuraliNS, Teramura AH.1986. Interspecific differences in Cucumis sativus sensitivity to ultraviolet‐B radiation. Physiologia Plantarum 68: 673–677. [Google Scholar]
- MusilCF,Bhagwandin N.1992. The SUN program for computation of solar ultraviolet spectral irradiances: solar exposure limits in South Africa. South African Journal of Science 88: 406–410. [Google Scholar]
- MusilCF, Wand SJE.1993. Responses of sclerophyllous Ericaceae to enhanced levels of ultraviolet‐B radiation. Environmental and Experimental Botany 33: 233–242. [Google Scholar]
- MusilCF, Midgely GF, Wand SJE.1999. Carryover of enhanced UV‐B exposure effects to successive generations of a desert annual: interaction with atmospheric CO2 and nutrient supply. Global Change Biology 5: 311–332. [Google Scholar]
- NewshamKK, McLeod AR, Greenslade PD, Emmett BA.1996. Appropriate controls in outdoor UV‐B supplementation experiments. Global Change Biology 2: 319–324. [Google Scholar]
- ReedHE, Teramura AH, Kenworthy WJ.1992. Ancestral U.S. Soybean cultivars characterized for tolerance to ultraviolet‐B radiation. Crop Science 32: 1214–1219. [Google Scholar]
- RozemaJ, van de Staaij J, Bjorn LO, Caldwell MM.1997. UV‐B as an environmental factor in plant life: stress and regulation. Trends in Ecology and Evolution 12: 22–28. [DOI] [PubMed] [Google Scholar]
- SantosI, Almeida JM, Salema R.1993. Plants of Zea mays L. developed under enhanced UV‐B radiation. Some ultrastructural and biochemical aspects. Journal of Plant Physiology 141: 450–456. [Google Scholar]
- SeckmeyerG, Mayer B, Erb R, Bernhard G.1994. UV‐B in Germany higher in 1993 than in 1992. Geophysical Research Letters 21: 577–580. [Google Scholar]
- SeckmeyerG, Mayer B, Bernhard G, McEnzie RL, Johnston PV, Kotkamp M, Booth CR, Lucas T, Mestechkina T, Roy CR, Gies HP, Tomlinson D.1995. Geographical differences in the UV measured by intercompared spectroradiometers. Geophysical Research Letters 22: 1889–1892. [Google Scholar]
- SheahanJJ.1996. Sinapate esters provide greater UV‐B attenuation than flavonoids in Arabidopsis thaliana (Brassicaceae). American Journal of Botany 83: 679–686. [Google Scholar]
- SmithGJ, Markham KR.1998. Tautomerism of flavonol glucosides – relevance to plant UV protection and flower colour. Journal of Photochemistry and Photobiology A – Chemistry 118: 99–105. [Google Scholar]
- SmithJL, Burritt DJ, Bannister P.2000. Shoot dry weight, chlorophyll and UV‐B‐absorbing compounds as indicators of a plant’s sensitivity to UV‐B radiation. Annals of Botany 86: 1057–1063. [Google Scholar]
- StapletonAE, Walbot V.1994. Flavonoids can protect maize DNA from the induction of ultraviolet radiation damage. Plant Physiology 105: 881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StittM.1991. Rising CO2 levels and their potential significance for carbon flow in plant cells. Plant Cell and Environment 14: 741–762. [Google Scholar]
- StridA, Porra RJ.1992. Alterations in pigment content in leaves of Pisum sativum after exposure to supplementary UV‐B. Plant Cell Physiology 33: 1015–1023. [Google Scholar]
- StridA, Chow WS, Anderson JM.1994. UV‐B damage and protection at the molecular level in plants. Photosynthesis Research 39: 475–489. [DOI] [PubMed] [Google Scholar]
- SullivanJH.1997. Effects of increasing UV‐B radiation and atmospheric CO2 on photosynthesis and growth: implications for terrestrial ecosystems. Plant Ecology 128: 194–206. [Google Scholar]
- SullivanJH, Teramura AH.1990. Field study of the interaction between solar ultraviolet‐B radiation and drought on photosynthesis and growth in soybean. Plant Physiology 92: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SullivanJH, Teramura AH, Ziska LH.1992. Variation in UV‐B sensitivity in plants from a 3000‐m elevation gradient in Hawaii. American Journal of Botany 79: 737–743. [Google Scholar]
- TeramuraAH.1983. Effects of ultraviolet‐B radiation on the growth and yield of crop plants. Physiologia Plantarum 58: 415–427. [Google Scholar]
- TeramuraAH, Sullivan JH.1987. Soybean growth responses to enhanced levels of ultraviolet‐B radiation under greenhouse conditions. American Journal of Botany 74: 975–979. [Google Scholar]
- TeramuraAH, Sullivan JH.1994. Effects of UV‐B radiation on photosynthesis and growth of terrestrial plants. Photosynthesis Research 39: 463–473. [DOI] [PubMed] [Google Scholar]
- TeramuraAH, Sullivan JH, Lyndon J.1990. Effects of UV‐B radiation on soybean yield and seed quality: A six‐year field study. Physiologia Plantarum 80: 5–11. [Google Scholar]
- TeviniM, Braun J, Fieser G.1991. The protective function of the epidermal layer of rye seedlings against ultraviolet‐B radiation. Photochemistry and Photobiology 53: 329–334. [Google Scholar]
- WandSJE, Midgley GF, Musil CF.1996. Physiological and growth responses of two African species, Acacia karoo and Themeda triandra, to combined increases in CO2 and UV‐B radiation. Physiologia Plantarum 98: 882–890. [Google Scholar]
- ZiskaLH, Teramura AH, Sullivan JH.1992. Physiological sensitivity of plants along an elevational gradient to UV‐B radiation. American Journal of Botany 79: 863–871. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


