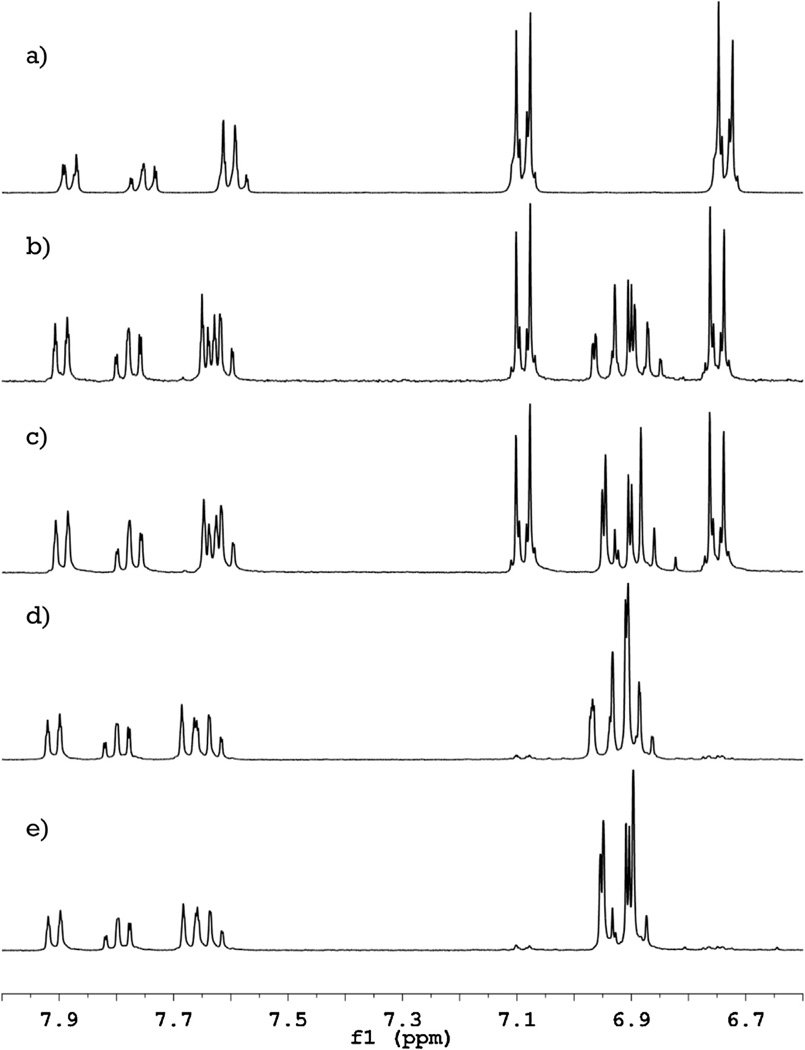
Fig. 1.
1H NMR spectra of phenolphthalein and its fluorinated derivatives. Successive introduction of fluorine atoms into the phenolphthalein molecule (a) causes only a minor shift of the signals from the carboxylic ring (δ 7.6–7.9 ppm) and gradual replacement of the signals from phenolic rings (δ 6.7 and 7.1 ppm) by a new group of peaks (δ 6.8–7.0 ppm) for mono- (b) and difluorophenolphthalein (d). Decoupling of the fluorine-hydrogen coupling (spectra (c) and (e) for mono- and difluorophenolphthalein, respectively) shows, that these new peaks are coupled with fluorine and allows an assignment of the proton signals.