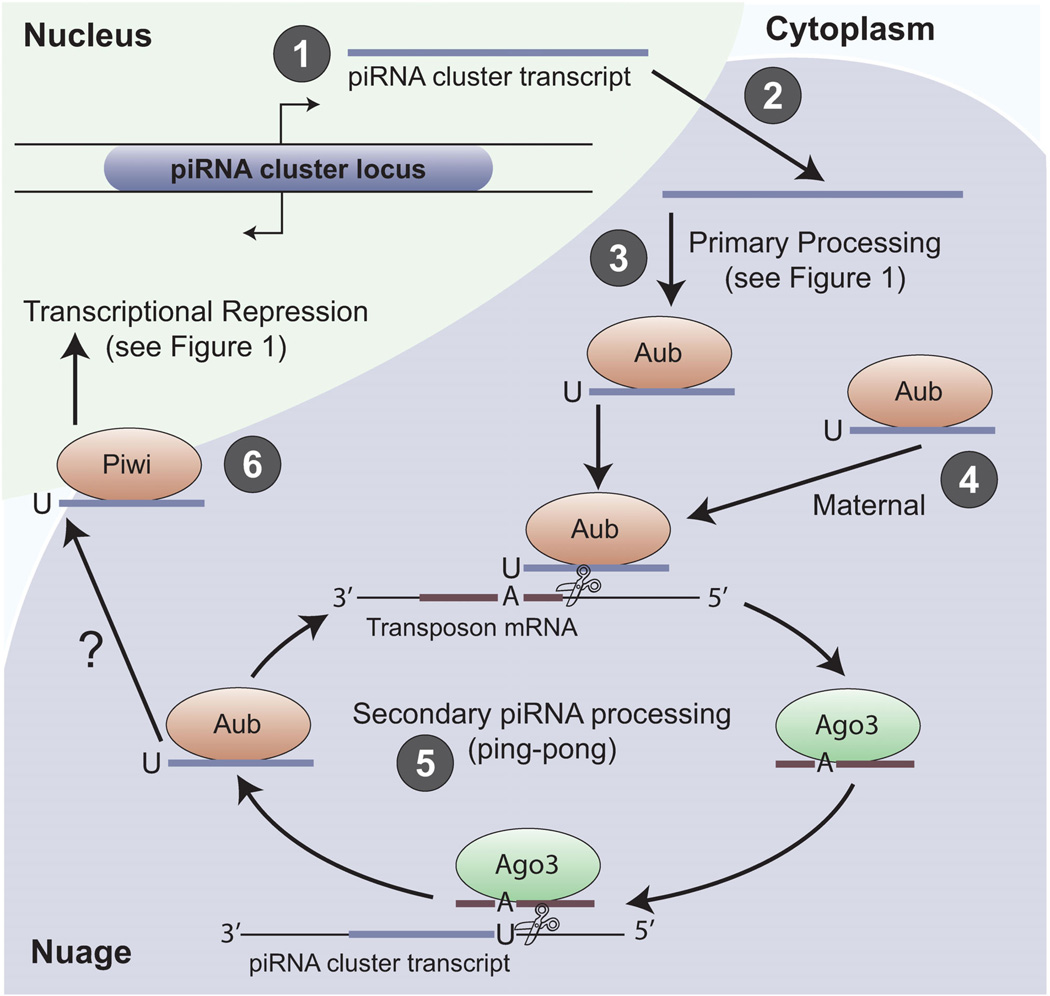
Figure 2.
Secondary piRNA processing in Drosophila ovarian germ cells. The mechanism of secondary piRNA biogenesis is best understood in Drosophila germ cells, but the signatures of this mechanism have been observed in animals from sponges to mice (see Table 2). Processing occurs as follows: (1) Primary transcripts are synthesized from piRNA cluster loci, which have bidirectional promoters in Drosophila female germ cells. Primary transcripts consist largely of dead transposon sequences oriented in both the sense and antisense directions. (2) Transcripts are exported and (3) processed into primary piRNAs that are antisense to active transposons (see Fig. 1). In Drosophila, both Piwi and Aub are capable of binding primary piRNAs. Secondary piRNA processing (also called ping-pong processing) requires the input of mature Aub/piRNA complexes from either primary processing or from (4) maternal contribution. The relative importance of these two sources of PIWI/piRNA complexes is not understood, and may depend on the type of transposon being silenced. (5) Aub/piRNA complexes bind to active transposon mRNAs, resulting in cleavage to form the 5′-end of a new piRNA; this slicing activity of PIWI proteins has only been shown in vitro. It is unknown how the 3′-end of the piRNA is formed, but it likely occurs by 3′-end trimming similar to primary piRNA processing. The newly formed piRNA is sense to active transposon mRNAs and bound by Ago3. This complex can direct the formation of new piRNAs from piRNA cluster loci, thus reinforcing the antisense nature of piRNAs bound to Aub. (6) Primary piRNAs bound to Piwi are transported into the nucleus to transcriptionally silence transposons, similar to Figure 1, but it is unclear if these piRNAs are made from ping-pong processing or solely from primary piRNA biogenesis.