Abstract
Aims/Introduction
Elevation of 2-h plasma glucose (2-h PG) levels keeps step with fasting plasma glucose (FPG) levels elevation, but some individuals show dominant elevation of 2-h PG and others FPG. We analyzed dependent and independent relationships between 2-h PG and FPG, and investigated the factors regulating 2-h PG and FPG.
Materials and Methods
In 1,657 Japanese participants who underwent a 75-g oral glucose tolerance test at the initial examination for a medical check-up, we carried out simple linear regression analysis between 2-h PG and FPG levels on the three patterns of independent variables. We divided the participants into two subgroups: the 2-h PG-side group and the FPG-side from the regression line, and examined the relationships between 2-h PG-FPG and factors responsible for elevation of plasma glucose levels.
Results
There was a significant positive correlation between 2-h PG and FPG levels. The regression line of both 2-h PG and FPG as independent variables was in accordance with the regression line of 2-h PG as an independent variable and FPG as a dependent variable. In 2-h PG-side group, age was the independent factor affecting 2-h PG in addition to insulinogenic index and insulin sensitivity index (ISI composite). In the FPG-side group, triglyceride was the independent factor affecting FPG in addition to insulinogenic index and ISI composite.
Conclusions
Two-hour PG was an independent predictor of FPG. In addition to the importance of decreased insulin secretion and insulin sensitivity, age was the strong factor to elevate 2-h PG levels in the 2-h PG-side group and triglyceride was the strong factor to elevate FPG levels in the FPG-side group in the early stage of development of type 2 diabetes.
Keywords: Fasting plasma glucose, Post-challenge plasma glucose, Type 2 diabetes
Introduction
Diagnosis of diabetes is based on 2-h plasma glucose (2-h PG) and fasting plasma glucose (FPG) levels during a 75-g oral glucose tolerance test (OGTT). Elevation of 2-h PG levels keeps step with FPG levels elevation; however, individuals showing dominant elevation of 2-h PG levels and showing dominant elevation of FPG levels exist. In addition, subjects with borderline hyperglycemia are categorized as impaired glucose tolerance (IGT), and subjects with impaired fasting glucose (IFG) are reported to have different pathophysiologies and phenotypes1,2. As IGT and IFG are reported to have a different incidence of the development of diabetes and microvascular complications3–6, hyperglycemic status in view of 2-h PG and FPG levels shows a wide variety of pathophysiology, development of diabetes and complications.
Type 2 diabetes consists of two main factors: decreased insulin secretion and insulin sensitivity. There is a significant linear relationship between 2-h PG and FPG levels, but there is also a mechanism to regulate differently. It is still controversial as to which factor is responsible for the elevation of 2-h PG and FPG levels. Some researchers described various factors, such as insulin secretory capacity, insulin resistance, age, body mass index (BMI), triglycerides and ethnicity, that influenced the elevation of 2-h PG levels1,2,7–13. In contrast, insulin resistance, decreased insulin secretory capacity, BMI, ethnicity and triglycerides were reported as factors influencing the elevation of FPG levels2,7–10,13,14.
Elevation of 2-h PG levels is an important factor, having a significant impact on cardiovascular disease (CVD) risk of patients with type 2 diabetes and borderline hyperglycemia. Large population studies, such as DECODE, Funagata and the DECODA study3–5, reported that post-challenge blood glucose levels are recognized to be crucial because IGT patients have a higher risk of CVD and mortality than IFG patients. Recently, Ning et al.6 reported that elevated 2-h PG level is associated with increased CVD mortality within the normoglycemic range in Europeans. Therefore, investigation of the factors elevating 2-h PG levels will be helpful to understand the pathophysiology and preventive strategies of diabetes and cardiovascular complications.
In the present study, we analyzed the relationship of 2-h PG and FPG levels using mathematical analysis. We investigated the dependent and independent relationships between 2-h PG and FPG levels from the OGTT examinations, and the mechanism regulating 2-h PG and FPG levels. We analyzed the factors responsible for elevation of 2-h PG and FPG levels dividing participants into two subgroups: dominant elevation of 2-h PG levels and dominant elevation of FPG levels.
Methods
Participants
We obtained clinical data from 1,657 participants who underwent 75-g OGTT owing to a positive urine glucose test; >5.5% glycated hemoglobin (HbA1c) level; family history of diabetes at initial examination for medical check-up at Kyoto University Hospital, Ikeda Hospital, Kansai Electric Power Hospital, Kansai Health Management Center, Center for Preventive Medicine of St. Luke's International Hospital and Kyoto Preventive Medical Center from 1993 to 2011. We excluded data from patients with hypertension, hepatic or renal dysfunction, endocrine or malignant disease, and a history of heavy exercise, gastrostomy or medication, which are known to affect glucose metabolism. Originally, 358 patients who had hypertension; hepatic, pancreatic or renal dysfunction; endocrine or malignant disease; or a history of heavy exercise, gastrectomy or medication known to affect glucose metabolism were excluded from the 2,193 patients. Among 1,853 patients, 196 patients were excluded because of FPG levels <60 mg/dL and >140 mg/dL or 2-h PG levels <60 mg/dL and >250 mg/dL for the present study to analyze the factors involved in the early stage of development of type 2 diabetes, and 1,657 patients were included. The study was designed in compliance with the ethics regulations of the Helsinki Declaration, and the study protocol was approved by the ethics committee of Okayama Prefectural University.
For measurement of plasma glucose and serum insulin during OGTT, we obtained fasting, 0.5-h, 1-h, 1.5-h and 2-h blood samples after oral administration of 75-g glucose. Standard OGTT with 75-g glucose was administered according to the National Diabetes Data Group recommendations, which requires subjects to fast overnight for 10–16 h before blood collection15. We measured HbA1c, triglyceride (TG), total cholesterol and high-density cholesterol (HDL-cholesterol) levels at fasting samples.
Laboratory Procedures
We measured plasma glucose and serum insulin levels in the blood samples during OGTT. Plasma glucose level was determined by the glucose oxidase method using a Hitachi Automatic Clinical Analyzer 7170 (Hitachi Co. Ltd., Tokyo, Japan). Serum insulin level was measured by chemiluminescent immunoassay (ARCHITECT insulin assay; Abbot Laboratories, Abbot Park, IL, USA). Serum total cholesterol and TG levels were measured as reported previously16. HbA1c was measured by HLC-723G7 (Tosoh Corp., Tokyo, Japan); and the HbA1c value, estimated as a National Glycohemoglobin Standardization Program equivalent value, was calculated by the formula: HbA1c (Japan Diabetes Society) +0.4% following the previous Japanese standard measurement methods17. Early insulin secretion was calculated using the formula for insulinogenic index: (insulin0.5 – insulin0 [pmol/L]) / (glucose0.5 – glucose0 [mmol/L])18. Insulin sensitivity was evaluated by insulin sensitivity index (ISI) composite: 10,000 / ([glucose0 × insulin0] × [mean glucose0–2 × mean insulin0–2])19. Disposition index (DI) was expressed as the multiplex of the indices of insulin secretion and insulin sensitivity20.
Analytical Procedures and Statistical Analyses
We carried out simple linear regression analysis between 2-h PG levels on the x-axis and FPG levels on the y-axis. We drew a straight regression line based on the least squares method according to the three patterns of independent variables:
Analysis A: Minimize the distance parallel to the y-axis; 2-h PG levels as an independent variable and FPG levels as a dependent variable.
Analysis B: Minimize the distance parallel to the x-axis; 2-h PG levels as a dependent variable and FPG levels as an independent variable.
Analysis C: Minimize the vertical distance from the regression line; both 2-h PG and FPG levels as independent variables, which was drawn by the MATLAB system (Math Works, Natick, MA, USA).
As shown in Figure1a, the regression line A corresponds to analysis A, the regression line B corresponds to analysis B and the regression line C minimizes corresponds to Analysis C. Figure1b is a frame format illustrating the method for regression line A, line B and line C. Setting the regression line by the least squares method means that the attracting force in proportion to the distance from each plot represents the strength (drawing power) of the spring. Distance A means the distance from the regression line A in parallel with the y-axis, distance B means from the regression line B in parallel with the x-axis, and distance C means the vertical and shortest distance from the regression line C.
Figure 1.
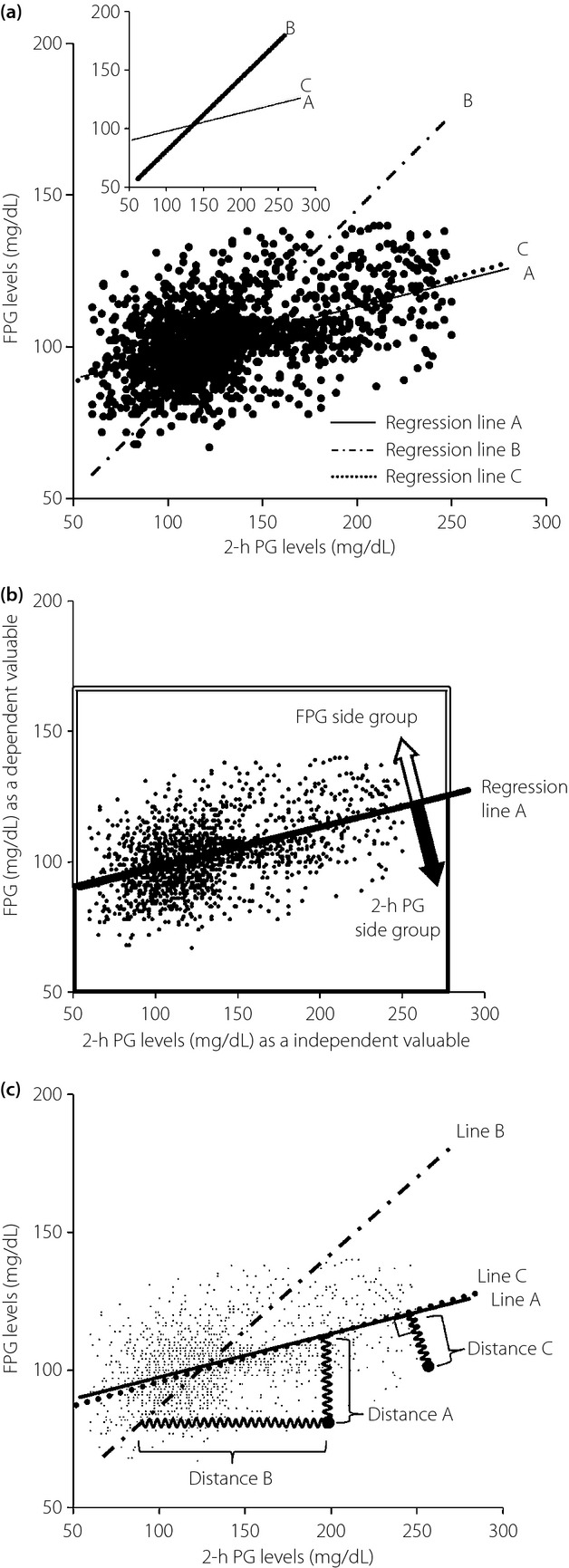
(a) Simple linear regression analysis between 2-h plasma glucose (PG) and fasting plasma glucose (FPG) levels. The solid line (regression line A) indicates a regression line of 2-h PG levels as an independent variable, obtained by the least squares method minimizing the residual sum of squares; sum of distances from the regression line in parallel with the y-axis (y = 0.16x + 81.70). The dashed-dotted line (regression line B) indicates a regression line of FPG levels as an independent variable, obtained by the least squares method minimizing the sum of distances from the regression line in parallel with the x-axis (y = 0.62x + 20.80). The dotted line (regression line C) indicates a regression line of both 2-h PG and FPG levels as independent variables, obtained by the least squares method minimizing the sum of vertical distances from the regression line (y = 0.17x + 80.11). (b) Grouping of participants according to regression line A. The plots located below regression line A belong to the FPG side group, and the plots located above regression line A belong to the 2-h PG side group. According to the position of the plot, the plot located above the regression line belongs to the FPG side group shown by the large open arrow surrounded by a double line; in contrast, the plot located below the regression line belongs to the 2-h PG side group shown by the large closed arrow surrounded by a heavy line. (c) The view illustrates a frame format to show the method for regression line A, line B and line C. Setting the regression line by the least squares method means that the attracting force proportional to the distance from the each plots represents the strength (drawing power) of the spring. Distance A means the distance from regression line A in parallel with the y-axis, distance B means from regression line B in parallel with the x-axis and distance C means the vertical and shortest distance from regression line C.
We divided the participants into two groups by the linear regression line A; locating above (y-axis side) or below (x-axis side) the regression line, with 2-h PG levels as an independent variable in the analysis A. According to the position of the plot, the plot located above the regression line belongs to the FPG side group shown by the large open arrow surrounded by a double line; in contrast, the plot located below the regression line belongs to the 2-h PG side group shown by the large closed arrow surrounded by a heavy line as shown in Figure1c. We defined the FPG-side group as deviated to the y-axis, namely, the FPG-side; and the 2-h PG-side group as deviated to the x-axis, namely, the 2-h PG-side. To examine the clinical characteristics of the 2-h PG-side and FPG-side groups, we carried out an unpaired Student's t-test between the two groups. Simple linear regression analysis was carried out for all participants to investigate the associations between 2-h PG/FPG levels and the other clinical factors, such as age, BMI, plasma glucose level, serum insulin level, HbA1c, TG, total cholesterol, HDL-cholesterol, insulinogenic index and ISI composite. P < 0.05 was considered as statistically significant. We carried out multivariate regression analysis to estimate the factors responsible for elevation of 2-h PG and FPG levels. All other statistical analyses were carried out using SPSS version 14.0 (SPSS, Chicago, IL, USA). All data are shown presented as mean ± standard error.
Results
Clinical Characteristics of Participants
The number of participants was 1,657 in total; 954 had normal glucose tolerance (NGT), 525 had impaired glucose regulation (IGR) and 78 had diabetes mellitus (DM), according to World Health Organization criteria. The mean age of the participants was 52.8 ± 0.3 years, and BMI was 23.2 ± 0.1 kg/m2. Parameters for glucose metabolism for the mean FPG, 2-h PG levels and HbA1c were 102.4 ± 0.3 mg/dL, 131.0 ± 1.0 mg/dL, and 5.7 ± 0.02%, respectively.
Simple Linear Regression Analysis Between 2-hPG and FPG
Figure1a shows the simple linear regression analysis between 2-h PG (x-axis) and FPG (y-axis) levels. The series of formulas of the simple linear regression analysis are as follows: (a) analysis A: 2-h PG levels as an independent variable and FPG levels as a dependent variable; (ii) analysis B: 2-h PG levels as a dependent variable and FPG levels as an independent variable; and (iii) analysis C: both 2-h PG and FPG levels as independent variables.
Regression line A: y = 0.16x + 81.70
Regression line B: x = 1.61y – 33.39 (y = 0.62x + 20.80)
Regression line C: 0 = 0.17x – 0.99y + 78.97 (y = 0.17x + 80.11)
There were positive correlations between 2-h PG and FPG (A: r = 0.504, P < 0.0001, B: r = 0.504, P < 0.0001). The residual sum of squares was A: 1.195 × 105, B: 1.198 × 106, respectively.
Clinical Characteristics of participant Groups With 2-h PG-Side and FPG-Side Groups
Clinical and metabolic characteristics of 2-h PG-side (n = 885) and FPG-side (n = 802) groups from the regression line are listed in Table1. Age (54.5 ± 0.4 years vs 51.2 ± 0.5 years; P < 0.0001), BMI (23.6 ± 0.1 kg/m2 vs 22.8 ± 0.3 kg/m2; P < 0.0001), HbA1c (5.8 ± 0.02% vs 5.5 ± 0.02%; P < 0.0001) were higher in the FPG-side group than that in the 2-h PG-side group.
Table 1.
Clinical characteristics of participants
| Total | FPG-side group | 2-h PG-side group | P-value | |
|---|---|---|---|---|
| n | 1,657 | 802 | 855 | – |
| Age (years)** | 52.8 ± 0.3 | 54.5 ± 0.4 | 51.2 ± 0.5 | <0.0001 |
| BMI (kg/m2)** | 23.2 ± 0.1 | 23.6 ± 0.1 | 22.8 ± 0.1 | <0.0001 |
| FPG (mg/dL)** | 102.4 ± 0.3 | 111.1 ± 0.4 | 94.2 ± 0.3 | <0.0001 |
| 2-h PG (mg/dL) | 131.0 ± 1.0 | 130.6 ± 1.4 | 131.4 ± 1.3 | NS |
| Fasting insulin (μU/mL)* | 5.6 ± 0.1 | 5.9 ± 0.1 | 5.3 ± 0.1 | <0.001 |
| HbA1c (%)** | 5.7 ± 0.02 | 5.8 ± 0.02 | 5.5 ± 0.02 | <0.0001 |
| Triglycerides (mg/dL) | 123.0 ± 3 | 125.2 ± 4.2 | 120.7 ± 4.3 | NS |
| Total cholesterol (mg/dL) | 208.2 ± 1.1 | 207.7 ± 1.6 | 208.8 ± 1.6 | NS |
| HDL cholesterol (mg/dL) | 61.2 ± 0.6 | 61.1 ± 0.8 | 61.3 ± 0.9 | NS |
| Insulinogenic index†† | 0.35 ± 0.01 | 0.30 ± 0.01 | 0.39 ± 0.01 | <0.0001 |
| ISI composite†† | 8.26 ± 0.1 | 7.30 ± 0.13 | 9.17 ± 0.15 | <0.0001 |
| Disposition index†† | 2.63 ± 0.08 | 1.96 ± 0.08 | 3.26 ± 0.14 | <0.0001 |
Data are presented as mean ± standard error. Significant differences between the 2-h plasma glucose (PG)-side and fasting plasma glucose (FPG)-side groups, tested by unpaired t-test are:
P < 0.001,
P < 0.0001 (2-h PG-side <FPG-side),
P < 0.0001 (2-h PG-side >FPG-side). BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; ISI, insulin sensitivity index; NS, not significant.
Regression Analysis Between 2-h PG and Factors Responsible for Elevation of 2-h PG in the 2-h PG-Side Group
In the 2-h PG-side group (n = 855), insulinogenic index (r = −0.348, P < 0.0001; Figure2a) and ISI composite (r = −0.328, P < 0.0001; Figure2b) showed significant correlations with 2-h PG levels in simple linear regression analysis. Age significantly correlated with 2-h PG levels (r = 0.329, P < 0.0001; Figure2c). According to the multivariate regression analysis, age was a strong factor to predict 2-h PG levels (β = 0.211) in addition to insulinogenic index (β = −0.453) and ISI composite (β = –0.337; Table2).
Figure 2.
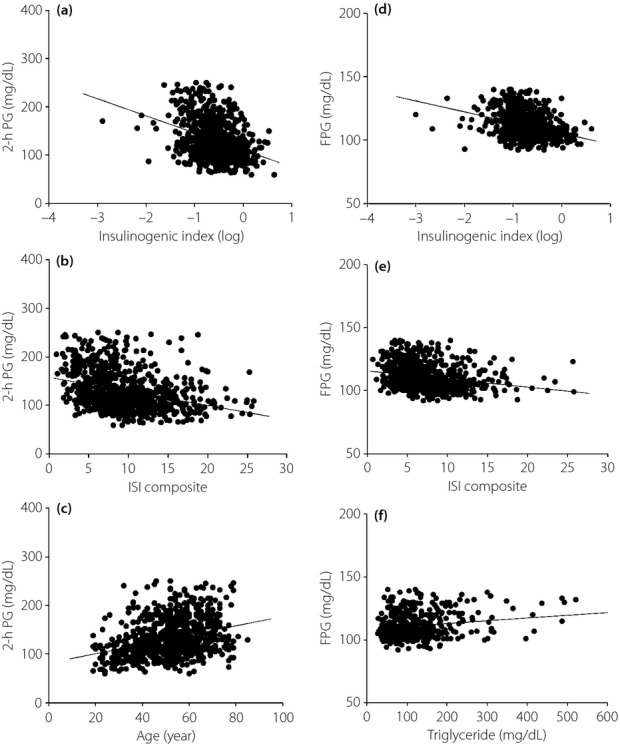
(a) The relationship between insulinogenic index (log) and 2-h plasma glucose (PG) in the 2-h PG-side group (r = −0.348, P < 0.0001). (b) The relationship between insulin sensitivity index (ISI) composite and 2-h PG in the 2-h PG-side group (r = −0.328, P < 0.0001). (c) The relationship between age and 2-h PG in the 2-h PG-side group (r = 0.329, P < 0.0001). (d) The relationship between insulinogenic index (log) and FPG in the FPG-side group (r = −0.347, P < 0.0001). (e) The relationship between ISI composite and FPG in the FPG-side group (r = −0.236, P < 0.0001). (f) The relationship between triglyceride and FPG in the FPG-side group (r = 0.206, P < 0.0001).
Table 2.
Relationship between 2-h plasma glucose and fasting plasma glucose and factors responsible for elevation of 2-h plasma glucose and fasting plasma glucose levels
| Variable | Multivariate regression analysis 2-h PG levels as a dependent variable | ||
|---|---|---|---|
| β-coefficients | Standard errors | P-value | |
| 2-h PG-side group from the regression line | |||
| Insulinogenic index (log) | −0.453 | 4.370 | <0.0001 |
| ISI composite | −0.337 | 0.423 | <0.0001 |
| Age | 0.211 | 0.125 | <0.0001 |
| BMI | 0.122 | 0.599 | <0.05 |
| TG | 0.101 | 0.017 | <0.05 |
| Variable | Multivariate regression analysis FPG levels as a dependent variable |
||
| β-coefficients | Standard errors | P-value | |
| FPG-side group from the regression line | |||
| Insulinogenic index (log) | −0.482 | 1.030 | <0.0001 |
| ISI composite | −0.359 | 0.129 | <0.0001 |
| TG | 0.101 | 0.004 | <0.05 |
| BMI | 0.083 | 0.127 | NS |
| Age | 0.06 | 0.037 | NS |
BMI, body mass index; ISI, insulin sensitivity index; PG, plasma glucose; TG, triglyceride.
Regression Analysis Between FPG and Factors Responsible for Elevation of FPG in FPG-Side Group
In the FPG-side group (n = 802), insulinogenic index (r =−0.374, P < 0.0001; Figure2d) and ISI composite (r = −0.236, P < 0.0001; Figure2e) significantly correlated with FPG levels in simple linear regression analysis. TG significantly correlated with FPG levels (r = 0.206, P < 0.0001; Figure2f). According to the multivariate regression analysis, TG was a strong factor to predict FPG levels (β = 0.101) in addition to insulinogenic index (β = −0.482) and ISI composite (β = −0.359; Table2).
Discussion
This is the first study to elucidate the dependent and independent relationship between 2-h PG and FPG levels mathematically, and analyze the causative factors of elevated 2-h PG and FPG levels. The regression line of both 2-h PG and FPG levels as independent variables was in accordance with the regression line of 2-h PG levels as an independent variable and FPG levels as a dependent variable, showing that 2-h PG level is an independent predictor of FPG level. In the 2-h PG-side group, we showed that age was the independent strong factor for predicting 2-h PG levels in addition to insulinogenic Index and ISI composite. In the FPG-side group, we found that TG was the strong factor for predicting FPG levels in addition to insulinogenic index and ISI composite. The differences of the mechanism to elevate 2-h PG and FPG levels are shown by dividing the participants into two subgroups from the regression line; 2-h PG level is associated with age and FPG level is associated with TG.
When we set 2-h PG levels as an independent variable and FPG levels as a dependent variable, the regression line followed the linear shape in the scatter plot. In contrast, when we set FPG levels as an independent variable and 2-h PG levels as a dependent variable, the slope of the regression line was oblique, as shown in Figure1, and the residual sum of squares was 10-fold as large as that of the regression line in which 2-h PG levels were independent (1.195 × 105 vs 1.198 × 106). The 2-h PG independent regression model fits to the scatter plot in comparison with the FPG independent regression model. When we set both 2-h PG and FPG levels as independent variables, the regression line approximated the line with 2-h PG as an independent variable and FPG as a dependent variable. These results showed that the 2-h PG level is an inherent independent variable for representing an individual's ability to reduce blood glucose levels after the administration of exogenous glucose (i.e., glucose tolerance), and the FPG level is a dependent variable affected by a variety of factors in addition to glucose tolerance.
To further analyze the factors responsible for elevation of 2-h PG in the 2-h PG-side and FPG in FPG-side group, we investigated the relationships between 2-h PG/FPG and the factors responsible for elevation of plasma glucose. In the 2-h PG-side group, setting 2-h PG as a dependent variable, we found age was an important factor next to insulinogenic index and ISI composite among the factors responsible for elevation of 2-h PG in multivariate regression analysis. Thus, it is considered that age was a strong factor affecting 2-h PG in addition to insulin secretion and sensitivity in multivariate regression analysis. Qiao et al.21 reported that age was more strongly associated with IGT than with IFG in normal Europeans. Szoke et al.22 reported that insulin secretion decreases dependently on age linearly at a rate of 0.7% per year in NGT subjects evaluated by the hyperglycemic clamp. They also described IGT subjects showing a larger decrease in insulin secretion compared with NGT subjects22. Bando et al.23 reported that the 2-h PG levels are strongly determined by age compared with FPG in Japanese subjects. Together with these observations, aging is associated with β-cell dysfunction and decreased insulin secretion, followed by 2-h PG elevation.
In the FPG-side group, setting FPG as a dependent variable, we found that TG was important next to insulinogenic index and ISI composite among the factors responsible for elevation of FPG in multivariate regression analysis. Thus, it is considered that TG was a strong factor for affecting FPG in addition to insulinogenic index and ISI composite. We previously reported that serum TG levels per se are associated with insulin action, and bezafibrate significantly improved TG levels, insulin resistance and blood glucose control in patients with diabetes24–26. It is considered that hypertriglyceridemia is associated with the elevation of FPG levels, and the reduction of serum TG levels improves insulin sensitivity and FPG elevation.
Insulinogenic index was the strong determinant responsible for 2-h PG and FPG levels in both the 2-h PG-side and FPG-side groups in the present study. It is still controversial as to whether decreased insulin secretory capacity or insulin sensitivity is the primary factor for elevating plasma glucose levels. Decreased insulin secretory capacity had a stronger effect to 2-h PG elevation in the studies of Japanese, Korean and Chinese subjects11,12,27–30, whereas decreased insulin sensitivity had a stronger involvement in 2-h PG elevation in the studies of Pima Indian, American, Finnish and Caucasian studies2,31–33. As there are ethnic differences in the contribution of insulin secretory capacity and insulin sensitivity to plasma glucose elevation and glucose intolerance as documented previously, further studies are required to establish whether similar results are observed in other ethnic populations.
The reason for differences of metabolic characteristics between the 2-h PG side group and the FPG side group in the present study is not known at present. To compare the difference of pathophysiology between both groups, it is necessary to compare the groups to include showing the dominant elevation of only FPG levels (such as isolated-IFG) and showing the dominant elevation of only 2-h PG levels (such as isolated-IGT). In addition, a longitudinal study is necessary to show how each group will deteriorate to diabetes, respectively.
We have elucidated 2-h PG levels as an independent predictor of FPG levels. We found that 2-h PG is an inherent value representing the ability to reduce blood glucose levels after the administration of exogenous glucose (i.e., glucose tolerance), and FPG levels are the result of regulation by complex factors in addition to factors affecting glucose tolerance. Age was associated with elevation of 2-h PG levels in the 2-h PG-side group and TG was associated with elevation of FPG levels in the FPG-side group, in addition to decreased insulin secretion and insulin sensitivity. These observations will be helpful for the prevention and treatment in the early stage of development of type 2 diabetes under the consideration of the pathophysiology and phenotype of each individual.
Acknowledgments
This research was supported by a Research Grant from the Japan Diabetes Society, Japan Association for Diabetes Education and Care, Manpei Suzuki Diabetes Foundation and Supporting Organization of Clinical Research on Life Style Related Disease, Leading Project of Biosimulation, Kobe Translational Research Cluster, and the Knowledge Cluster Initiative from the Ministry of Education, Culture, Sports, Science and Technology, Japan. The authors do not have any financial support or relationships that pose a conflict of interest. We thank Kobe Industrial Health Association, Kyoto Industrial Health Association, Fukuoka Institute of Occupational Health, Morinomiyako Occupational Health Association, Niigata Association of Occupational Health and Hokkaido Industrial Health Association for their help in the study.
References
- Hanefeld M, Koehler C, Fuecker K, et al. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2003;26:868–874. doi: 10.2337/diacare.26.3.868. Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. [DOI] [PubMed] [Google Scholar]
- Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 1999;48:2197–2203. doi: 10.2337/diabetes.48.11.2197. [DOI] [PubMed] [Google Scholar]
- DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Eguchi H, Manaka H, et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- Nakagami T DECODA study Group. Hyperglycemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004;47:385–394. doi: 10.1007/s00125-004-1334-6. [DOI] [PubMed] [Google Scholar]
- Ning F, Tuomilehto J, Pyörälä K, et al. DECODE Study Group. Cardiovascular disease mortality in Europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diabetes Care. 2010;33:2211–2216. doi: 10.2337/dc09-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Q, Hu G, Tuomilehto J, et al. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care. 2003;26:1770–1780. doi: 10.2337/diacare.26.6.1770. [DOI] [PubMed] [Google Scholar]
- The DECODE Study Group. Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care. 2003;26:61–69. doi: 10.2337/diacare.26.1.61. [DOI] [PubMed] [Google Scholar]
- Meyer C, Pimenta W, Woerle HJ, et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29:1909–1914. doi: 10.2337/dc06-0438. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance. Implications for care. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Fukushima M, Usami M, et al. Factors responsible for development from normal glucose tolerance to isolated post-challenge hyperglycemia. Diabetes Care. 2003;26:1211–1215. doi: 10.2337/diacare.26.4.1211. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004;53:831–835. doi: 10.1016/j.metabol.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Mitsui R, Fukushima M, Nishi Y, et al. Factors responsible for deteriorating glucose tolerance in newly diagnosed type 2 diabetes in Japanese men. Metabolism. 2006;55:53–58. doi: 10.1016/j.metabol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Fukushima M, Suzuki H, et al. Insulin secretion and insulin sensitivity in Japanese subjects with impaired fasting glucose and isolated fasting hyperglycemia. Diabetes Res Clin Pract. 2005;70:46–52. doi: 10.1016/j.diabres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- Taniguchi A, Fukushima M, Sakai M, et al. Remnant-like particle cholesterol, triglycerides, and insulin resistance in non-obese Japanese type 2 diabetic patients. Diabetes Care. 2000;23:1766–1769. doi: 10.2337/diacare.23.12.1766. [DOI] [PubMed] [Google Scholar]
- Seino Y, Nanjo K, Tajima N, et al. The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Ikeda M, Yawata M, et al. The insulinogenic index in secondary diabetes. Horm Metab Res. 1975;7:107–115. [Google Scholar]
- Matsuda M, Defronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euroglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Yokota K, Fukushima M, Takahashi Y, et al. Insulin secretion and computed tomography values of pancreas in the early stage of the development of diabetes. J Diabetes Invest. 2012;3:371–376. doi: 10.1111/j.2040-1124.2012.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Q, Tuomilehto J, Balkau B, et al. DECODE Study Group. Are insulin resistance, impaired fasting glucose and impaired glucose tolerance all equally strongly related to age? Diabet Med. 2005;22:1476–1481. doi: 10.1111/j.1464-5491.2005.01655.x. [DOI] [PubMed] [Google Scholar]
- Szoke E, Shrayyef MZ, Messing S, et al. Effect of Aging on glucose homeostasis: accelerated deterioration of β-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31:539–543. doi: 10.2337/dc07-1443. [DOI] [PubMed] [Google Scholar]
- Bando Y, Ushiogi Y, Okafuji K, et al. The relationship of fasting plasma glucose values and other variables to 2-h postload plasma glucose in Japanese subjects. Diabetes Care. 2001;24:1156–1160. doi: 10.2337/diacare.24.7.1156. [DOI] [PubMed] [Google Scholar]
- Taniguchi A, Fukushima M, Sakai M, et al. Effects of bezafibrate on insulin sensitivity and insulin secretion in non-obese Japanese type 2 diabetic patients. Metabolism. 2001;50:477–480. doi: 10.1053/meta.2001.21028. [DOI] [PubMed] [Google Scholar]
- Taniguchi A, Fukushima M, Sakai M, et al. The role of the body mass index and triglyceride levels in identifying insulin-sensitive and insulin-resistant variants in Japanese non-insulin-dependent diabetic patients. Metabolism. 2000;49:1001–1005. doi: 10.1053/meta.2000.7735. [DOI] [PubMed] [Google Scholar]
- Teramoto T, Shirai K, Daida H, et al. Effects of bezafibrate on lipid and glucose metabolism in dyslipidemic patients with diabetes: the J-BENEFIT study. Cardiovasc Diabetol. 2012;11:29. doi: 10.1186/1475-2840-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66S:37–43. doi: 10.1016/j.diabres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Oka R, Yagi K, Sakurai M, et al. Insulin secretion and insulin sensitivity on the oral glucose tolerance test (OGTT) in middle-aged Japanese. Endocr J. 2012;59:55–64. doi: 10.1507/endocrj.ej11-0157. [DOI] [PubMed] [Google Scholar]
- Rhee SY, Woo JT, Chon S, et al. Characteristics of insulin resistance and insulin secretory capacity in Korean subjects with IFG and IGT. Diabetes Res Clin Pract. 2010;89:250–255. doi: 10.1016/j.diabres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Qian L, Xu L, Wang X, et al. Early insulin secretion failure leads to diabetes in Chinese subjects with impaired glucose regulation. Diabetes Metab Res Rev. 2009;25:144–149. doi: 10.1002/dmrr.922. [DOI] [PubMed] [Google Scholar]
- Stancáková A, Javorský M, Kuulasmaa T, et al. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes. 2009;58:1212–1221. doi: 10.2337/db08-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa A, D'Agostino R, Jr, Hanley AJ, et al. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes. 2004;53:1549–1555. doi: 10.2337/diabetes.53.6.1549. [DOI] [PubMed] [Google Scholar]
- Carnevale SG, Rossi A, Sainaghi PP, et al. The significance of impaired fasting glucose versus impaired glucose tolerance: importance of insulin secretion and resistance. Diabetes Care. 2003;26:1333–1337. doi: 10.2337/diacare.26.5.1333. [DOI] [PubMed] [Google Scholar]


