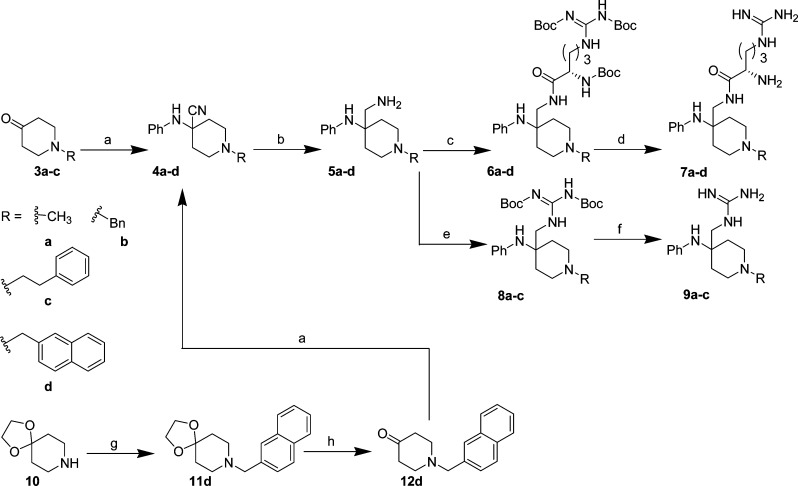
Scheme 1. Synthesis of 7a–d and 9a–c.
Reagents and conditions: (a) aniline, TMSCN, AcOH, 0 °C, 1 h, 70–94%; (b) LiAlH4, diethyl ether (ah), 0 °C, 35–40% (for 5a,5c) or NH3/MeOH, Raney Ni, 3 atm H2(g), 4 h, rt, 82–90% (for 5b, 5d); (c) Boc-Arg-(Boc)2-OH, EDCI, HOBt, Et3N, DCM, rt, 24 h, 50–70%; (d) HCl/dioxane, 3 days, rt, 95%; (e) 1,3-bis(tert-butoxycarbonyl)-2-methyl-2-thiopseudourea, HgCl2, Et3N, DMF, 0 °C → rt, 10 h, 30%; (f) HCl/dioxane, 4 days, rt, quantitative; (g) 2-bromomethylnaphthalene, K2CO3, KI, 4-methyl-2-pentanone, reflux, 5 h; (h) AcOH, conc. HCl (aq), reflux, 18 h, 40%.