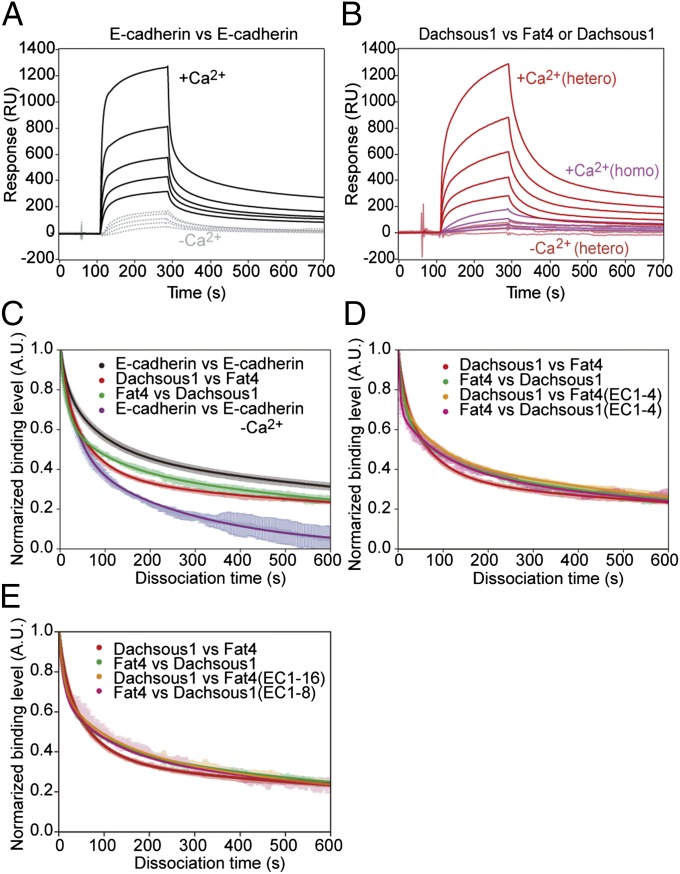
Fig. 4.
Heterophilic interactions between Fat4 and Dachsous1 via their N-terminal domains. (A and B) Surface plasmon resonance (SPR) analysis of the binding between E-cadherin ectodomains (A) or between Dachsous1 and Fat4 ectodomains (B, hetero) in the presence or absence of Ca2+. In B, the binding between Dachsous1 and Dachsous1 (homo) was also assayed. (C) Dissociation processes of E-cadherin from E-cadherin, Fat4 from Dachsous1, and Dachsous1 from Fat4. E-cadherin was analyzed also in the absence of Ca2+. (D and E) Comparisons between the dissociation processes of Fat4 from Dachsous1 (and vice versa), Dachsous1 fragments from Fat4, and Fat4 fragments from Dachsous1. Two fragments of Fat4, EC1-4 and EC1-16, and those of Dachsous1, EC1-4 and EC1-8, were used. In all of the molecular pairs shown by “vs.,” the one indicated at the left was adsorbed to the assay substrate, and that at the right was dissolved in the reaction solution.