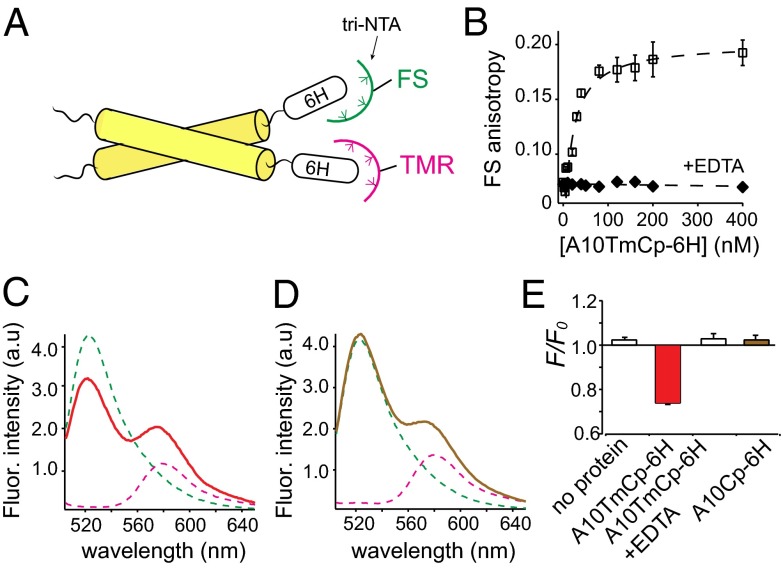
Fig. 3.
A10TmCp-6H, but not A10Cp-6H, forms an oligomer in DPC micelles. (A) Illustration of the FRET experiment. Fluorophore (FS, TMR)-conjugated tri-NTA noncovalently associates with the hexahistidine (6H) tag in the target protein. When the protein self-associates, FRET will occur between the bound FS and TMR. (B) Binding of A10TmCp-6H to FS-tri-NTA dissolved in the DPC micellar solution containing 1 µM NiSO4 monitored by FS fluorescence anisotropy. The binding curve was fitted to a hyperbolic binding equation with a binding affinity of 8 ± 2 nM (Table 1). A10TmCp-6H induced little change in FS anisotropy in the presence of 10 mM EDTA (filled diamond), indicating that FS-tri-NTA bound specifically to the 6H sequence in A10TmCp-6H. (C and D) Fluorescence emission spectra of 10 nM FS-tri-NTA (green), 100 nM TMR-tri-NTA (pink), and the mixture of both in complex with 200 nM A10TmCp-6H (red) or A10Cp-6H (brown) in DPC micelles. (E) Comparison of fluorescence quenching for the noted proteins that were added to the mixture of FS- and TMR-tri-NTA.