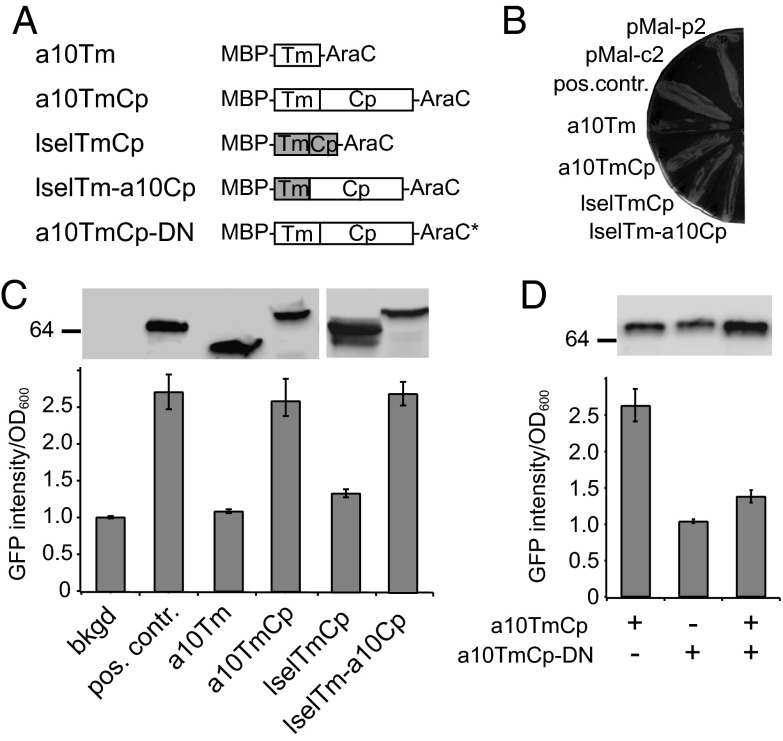
Fig. 5.
The cytoplasmic domain of ADAM10 dimerizes in a cell membrane. (A) Illustration of MBP-AraC constructs used in the study. Construct a10TmCp-DN contains the same ADAM10 residues as in a10TmCp plus a loss-of-function mutation in the AraC domain (AraC*). (B) MalE complementation to test the topology of chimeric proteins. The pMal-p2 and pMal-c2 plasmids express MBP protein in the periplasm and cytoplasm of E. coli, respectively. The positive control construct (pos. contr.) expresses the MBP-AraC chimeric protein containing a tightly dimerizing integrin αIIb transmembrane sequence (28). (C and D) Ratios of GFP fluorescence intensity vs. OD600 for each construct are compared with the background (bkgd) and positive control. In the background sample, E. coli was not transformed with the MBP-AraC–expressing vector. (Upper) Comparable expression levels of chimeric MBP-AraC proteins probed by Western blot using the anti-MBP antibody.