Fig. 3.
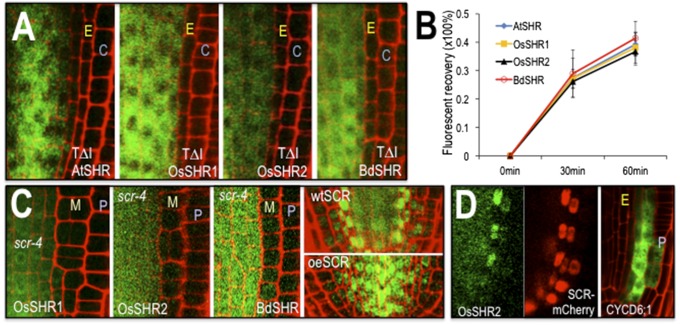
The SHR proteins have similar requirements for localization and movement. (A) Mutation of a conserved threonine (residue 289 in AtSHR, 345 in OsSHR1, 352 in OsSHR2, and 354 in BdSHR) in the VHIID domains of the SHR proteins inhibits nuclear localization and intercellular movement. C, cortex; E, endodermis. Full root tip images are in Fig. S2. (B) Quantification of fluorescence recovery after photobleaching (FRAP) showed that the kinetics of protein movement between the stele and the first ground tissue is comparable among all SHR homologs [n = 3 replicates, 9 roots and 35–132 cells for each SHR homolog; assays were done in wild type (WT)]. (C) The scr-4 mutation is epistatic to the effects of expression of the SHR homologs. M, mutant cell layer; P, epidermis. The first three panels show transgene expression from the SHR promoter in the scr-4 (null) background. The last two panels show OsSHR2-YFP in a WT root and a root overexpressing (oe) AtSCR-mTFP from the SHR promoter. (D) Expression of OsSHR2 from the SHR promoter (Left and Middle) drives expression of the SCR:mCherry marker and expression of CYCD6;1:erGFP (Right), which is visualized without the OsSHR2-YFP signal.