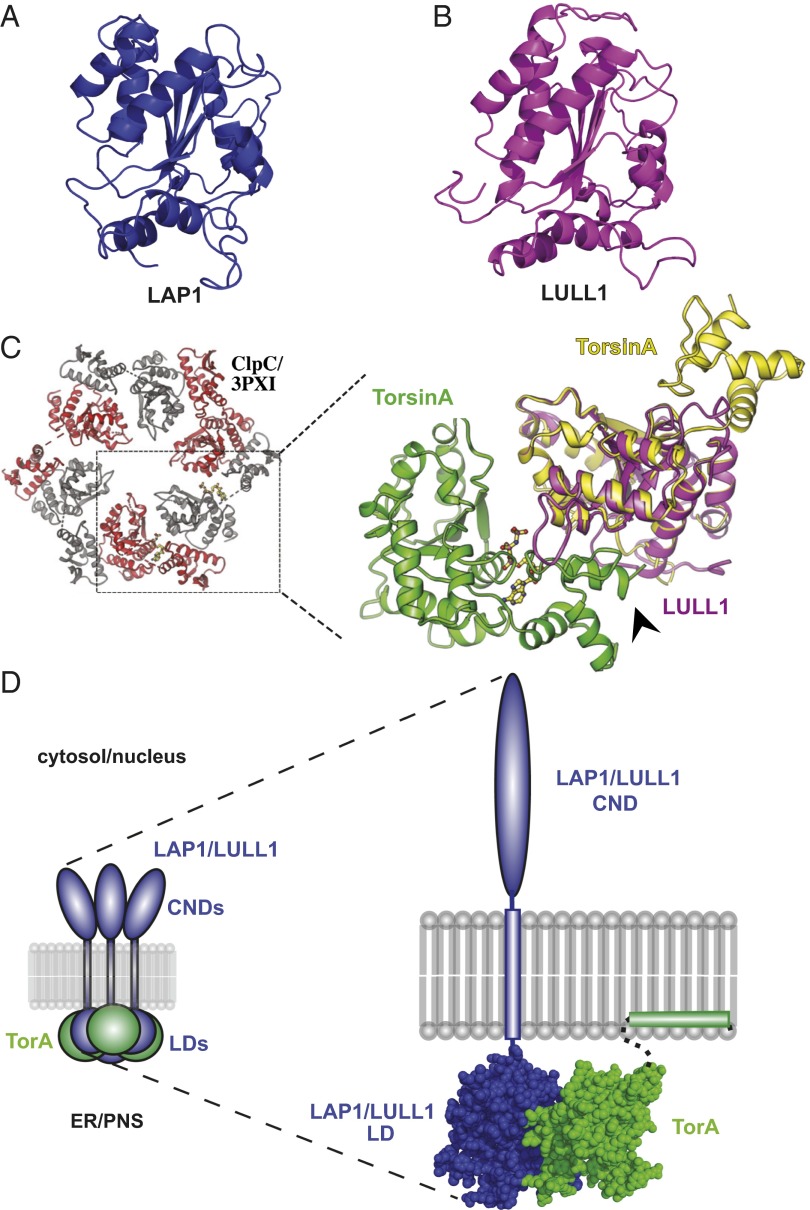
Fig. 1.
LAP1 and LULL1 luminal domains are predicted to adopt an AAA+-like fold. (A) Predicted structural model of LAP1’s luminal domain using Phyre2. (B) As in A, but for LULL1. (C) Oligomeric model of TorA (green and yellow) using a least-squares superposition of alpha-carbons (Coot) onto ClpC’s hexameric structure (Protein Data Bank ID code 3PXI) bound to adenylylimidodiphosphate (AMPPNP). A least-squares superposition of LULL1LD (magenta) onto a TorA monomer is shown. AMPPNP is colored by element. The arrowhead indicates TorA’s C terminus. (D, Left) Proposed mixed-ring assembly of LAP1/LULL1 (blue) with TorA (green). CNDs, cytoplasmic/nuclear domains; PNS, perinuclear space; LDs, luminal domains. (D, Right) Space-filling structural model of the LAP1/LULL1-TorA heterodimer. Regions not modeled are shown as dashed lines, membrane domains are shown as blocks, and the CND is shown as an ellipse.