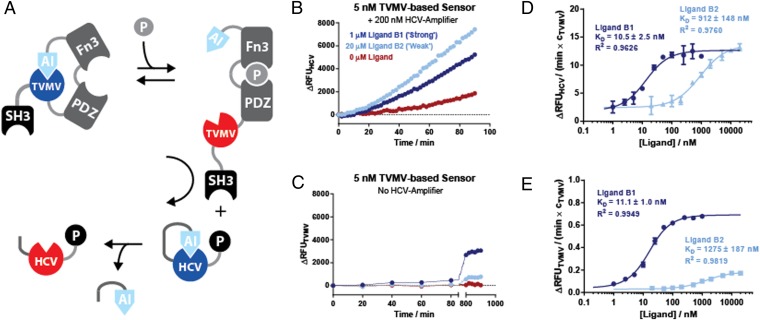
Fig. 3.
(A) Construction of an integrated signal sensing and amplification circuit from an allosterically regulated protease sensor TVMVE217-PDZG38-FN3GPG-AI and an HCV-based signal transducer. Efficient signal transmission between the sensor and the transducer is ensured by protein–peptide interactions between an SH3 domain and its cognate peptide ligand fused to the modules. (B) Activation of HCV protease activity in response to affinity clamp ligand peptides B1 and B2 in an integrated signaling circuit. (C) A control experiment where the protease activity of the TVMV affinity clamp chimera was measured directly in the presence or absence of the two peptide ligands B1 and B2. (D) Determination of apparent Kd values for B1 and B2 using experimental setup shown in B with different ligand concentrations. The rate of HCV activity in the exponential phase (normalized over the concentration of TVMV) was plotted against the concentration of the ligand peptide and fitted to Eq. S2. (E) As in D but directly measuring the activity of the TVMV-based receptor from experiment shown in C. RFU units generated through the cleavage of TVMV- and HCV-specific peptide protease substrates in the two different systems scale by a factor of 1.8. Protease activities in D and E are normalized over the concentration of the ligand sensing TVMV protease. Proteases were assayed with 5 μM quenched fluorescent substrate peptide.