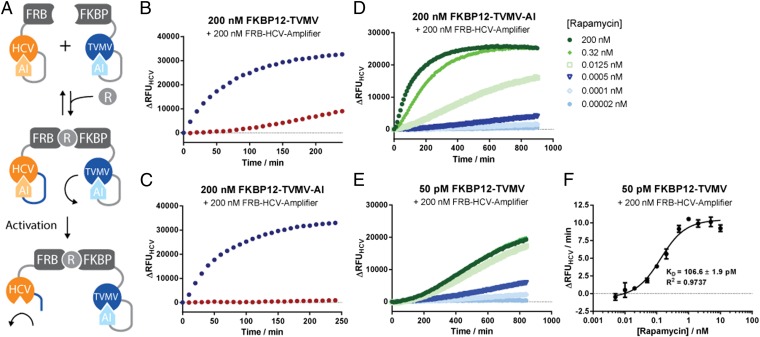
Fig. 4.
(A) Principle design of autoinhibited protease-based proximity sensors. (A) TVMV-inducible mutant of HCV was fused to FRB and a thrombin-inducible mutant of TVMV was fused to FKBP12. In the presence of rapamycin, TVMV colocalizes with HCV resulting in rapid cleavage of the latter. (B and C) Time-resolved traces of protease activities of HCV activity of the sensors in the absence (red) or presence (blue) of rapamycin. Note that background activation is significantly reduced in the presence of the AI domain (D and E). Titration of rapamycin to the two-component sensor induces HCV activation at subpicomolar concentrations. (F) Quantification of Kds for rapamycin for uninhibited TVMV at different ligand concentrations. Proteases were assayed with 5 μM quenched fluorescent substrate peptide.