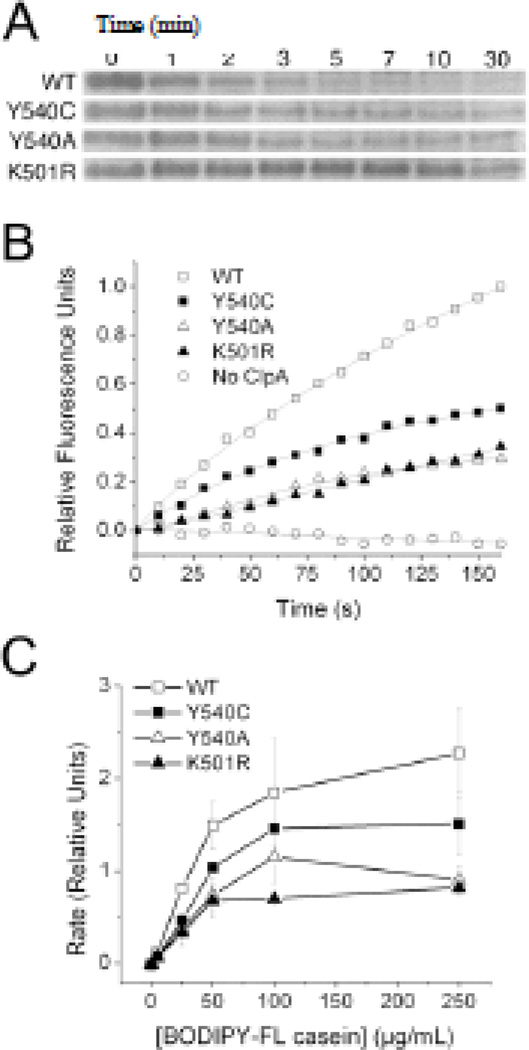
Figure 1.
Enzymatic Properties of ClpA D2 Loop Mutants. A) Casein bands from gel electrophoresis samples show that wild-type ClpAP degrades α-casein more rapidly than ClpA(Y540A/C)P mutants, which in turn process the substrate slightly faster than ClpA(K501R)P. Numbers above gel lanes indicate time in minutes at which samples were quenched. B) Fluorescence emission at 530 nm for reaction mixtures containing 250 µg/mL BODIPY FL casein; fluorescence increases upon substrate cleavage by ClpAP. The D2 loop mutants degrade the BODIPY FL casein substrate at a slower rate than does wild-type ClpA. C) Plot of rate as a function of substrate concentration for BODIPY FL casein degradation by wild-type and mutant ClpAP. Error bars are the standard error in the mean for three trials.