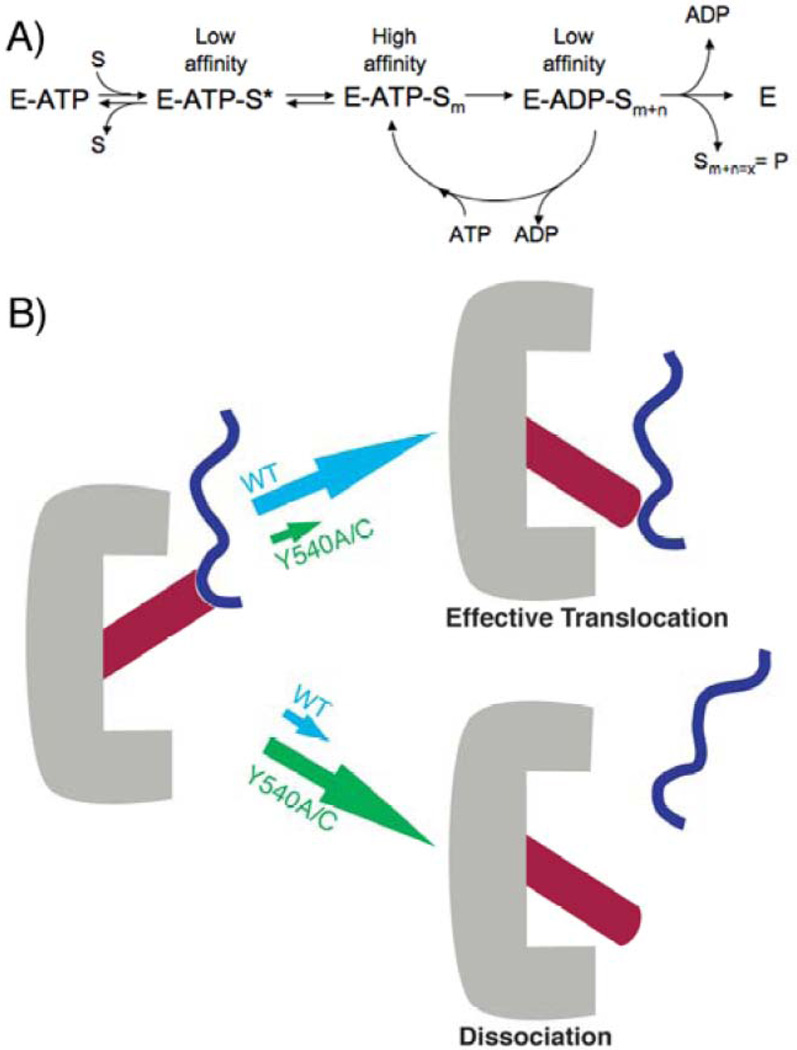
Figure 5.
Model for substrate processing. A) The enzyme (E) binds ATP and then substrate to form a putative pre-binding complex E-ATP-S* that is in equilibrium with a higher affinity, more tightly bound complex E-ATP-Sm. Active ATP hydrolysis drives an iterative cycling process. Some cycles will result in an increase in the position ‘m’ (i.e., n>0), translocating the substrate partially through the central pore. Other cycles will result in ineffective ATP hydrolysis, such that n=0 and the position m does not change (i.e., the substrate is not moved with respect to the pore). Product is released when the substrate has been completely translocated through the central pore. B) Cartoon depiction of loop movement in ClpA. The loop movement conformational change associated with ATP hydrolysis may be coupled to an effective translocation step for substrate, or the substrate may dissociate before the conformational change, preventing effective translocation. Partitioning between these two possibilities is a function of the stability of the pre-hydrolytic state (i.e., how tightly the loop holds on to substrate in the ATP-bound state).