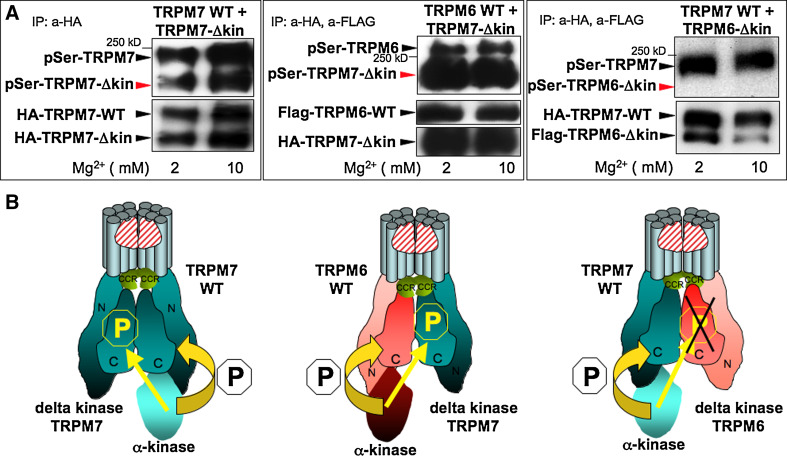
Fig. 1.
The channel-kinase TRPM6 phosphorylates serine residues of associated TRPM7 but not vice versa. Human HA-tagged TRPM7 WT, human HA-tagged TRPM7 delta kinase (TRPM7-Δkin), human flag-tagged TRPM6 WT, and human flag-tagged TRPM6 delta kinase (TRPM6-Δkin) have been cloned, stably transfected and inducibly co-expressed in HEK-293 cells as indicated, see methods section and reference [11] for further details. In vitro phosphorylation reactions have been performed after HA and/or Flag immunoprecipiations under two different Mg2+ concentrations (2 and 10 mM) with 100 μM MgATP [11]. Phosphorylation of these proteins was subsequently analyzed after gel electrophoresis and immunoblotting with a general anti phospho serine antibody. Protein expression levels were verified after stripping of the membrane via reprobing with anti FLAG or anti HA antibodies. a Left panel TRPM7 kinase crossphosphorylates TRPM7 delta kinase channels in vitro upon TRPM7 WT and TRPM7 delta kinase co-expression. Middle panel TRPM6 kinase crossphosphorylates TRPM7 delta kinase channels in vitro upon TRPM6 WT and TRPM7 delta kinase co-expression. Right panel TRPM7 kinase does not crossphosphorylate TRPM6 delta kinase channels in vitro upon TRPM7 WT and TRPM6 delta kinase co-expression. These data are representative of three separate experiments. b Based on our results, model showing how TRPM6 and TRPM7 form heteromers and crossphosphorylate each other. TRPM6 and TRPM7 have to tetramerize in order to build a functional ion channel pore