Abstract
Objective
Functional deficits seen in several neurodegenerative disorders have been linked with dysfunction in fronto-striatal circuits and with associated shape alterations in striatal structures. The severity of visible white matter changes (WMC) on MRI has been found to correlate with poorer performance on measures of gait and balance. This study aimed to determine whether striatal volume and shape changes were correlated with gait dysfunction.
Method
MRI scans and clinical gait/balance data (scores from the SPPB - Short Physical Performance Battery) were sourced from 66 subjects in the previously-published LADIS trial, which was performed in >65 y.o. non-disabled individuals with WMC at study entry. Data were obtained at study entry and at three-year follow-up. Caudate nuclei and putamina were manually traced using a previously published method, and volumes calculated. The relationships between volume and physical performance on the SPPB were investigated with shape analysis utilising the SPHARM toolkit.
Results
There was no correlation between the severity of WMC and striatal volumes. Caudate nuclei volume correlated with performance on the SPPB at baseline, but not at follow-up, with subsequent shape analysis showing regionalisation of left caudate changes in areas corresponding to inputs of the dorsolateral prefrontal, premotor and motor cortex. There was no correlation between putamen volumes and performance on the SPPB.
Conclusion
Disruption in frontostriatal circuits may play a role in mediating poorer physical performance in individuals with white matter changes. Striatal volume and shape changes may be suitable biomarkers for functional changes in this population.
Introduction
The Implications of White Matter Changes
White matter changes (WMC), defined as focal and confluent white matter hyperintensities on T2-weighted MRI brain scans, are particularly prominent in individuals over the age of 65 [1, 2]. Previous studies have examined the correlation of white matter changes and cognitive ability in this older age group, with the largest being the Leukoaraiosis and Disability Study (LADIS). The LADIS study is a multi-centre European study which investigated the effect of WMC on a number of different clinical indicators over a three-year prospective follow-up [3, 4]. The LADIS study has demonstrated a number of associations between severity of WMC and adverse outcomes, which included increased transition to disability [5], greater incidence of depression [6, 7], as well as declining cognitive ability [8-12].
The LADIS study also demonstrated a significant association between the severity of WMC/WMH and the prevalence of gait and balance disorders – both in the frequency of falls and in poorer scores obtained on scales such as the Short Physical Performance Battery (SPPB) [13, 14]. This is consistent with recent literature identifying gait and balance disorders in the elderly as a marker of other serious morbidity and mortality, particularly through proxy measures such as walking speed, which has been linked to all-cause and cardiovascular mortality [15, 16] as well as subsequent development of dementia [17].
Fronto-Striatal Circuits and Striatal Shape Analysis
The link between the basal ganglia of the brain and motor co-ordination and planning is well documented [18-20]. While basal ganglia volumes have been the mainstay of MRI studies, studies of three dimensional shape alterations in the striatal areas of the basal ganglia, particularly in the caudate nucleus, have been demonstrated in a number of studies examining different neurodegenerative disorders characterised by both motor abnormalities and dementia [21-25]. Our group has previously examined caudate nucleus volumetrics in patients with stroke and vascular dementia [26].
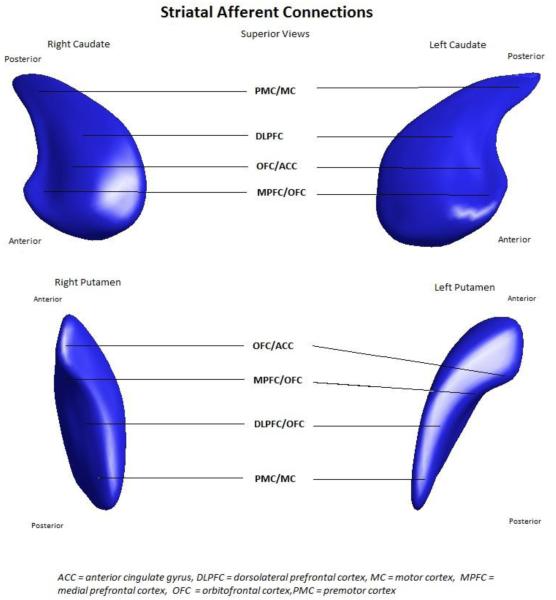
Afferents to the caudate arise from different areas of the cortex, making the caudate nucleus an important part of parallel fronto-striatal circuits [27]. This model, first described by Alexander et al, and confirmed through a number of different modalities [28-31], indicates that different areas of the cortex ‘map’ on to different areas of the caudate nucleus and putamen (Figure 1). Specifically, the cognitive and behavioural circuits arising from dorsolateral prefrontal cortex, anterior cingulate and orbitofrontal cortex primarily project to the caudate, while the frontal eye fields and motor cortex project to both caudate and putamen [27, 32]. Accordingly, functional changes in cognition, behaviour and movement may arise from structural change in caudate and putamen. Therefore, we hypothesized that motor function may be correlated with the structural integrity and hence morphology of the caudate and putamen and sought to examine this hypothesis through three dimensional shape analyses of these structures.
Figure 1.
Striatal afferent connections, showing the location on the caudate and putamen that each cortical region preferentially connects, based on the circuit diagrams from Alexander et al 1986.
This study aimed to determine whether subjects entering the LADIS study had striatal shape reductions - ‘deflation” - in specific areas at baseline, corresponding to the site of afferent connections from cortical motor circuits. Our chief hypothesis was that we would detect shape deflation in striatal regions corresponding to areas of the motor cortex, and that this deflation would correlate with poorer performance on the Short Physical Performance Battery. Gait, measured via walking speed in the SPPB, has been associated with cognitive impairment and progression to dementia [33-35]. We further hypothesised that striatal morphology would be associated with walking speed. Finally, as LADIS is a longitudinal study, we sought to investigate these structure-function relationships with clinical data at 3 year follow-up.
Methods
LADIS Study Data Collection and Details
The recruitment methods and other clinical data for LADIS have been documented elsewhere [3] in brief: 639 subjects were recruited from 11 centres in Europe: Florence, Helsinki, Graz, Lisboa, Amsterdam, Gothenburg, Huddinge, Paris, Mannheim, Copenhagen, and Newcastle-upon-Tyne. Inclusion criteria were an age range between 65 and 84 years, white matter changes as rated by the Fazekas Scale (as mild, moderate and severe) on T2 and FLAIR MRI [36] – during the course of the study. WMC were also assessed via total WMC volume and through the Scheltens scale [37] but the different measurement methods did not result in any differences in correlations with gait or balance outcomes [38]. The other criterion for entry was a score on the International Activities of Daily Living (IADL)[39] scale indicating no or mild disability. The exclusion criteria were the presence of severe medical or psychiatric illness, severe unrelated neurological diseases (specifically, this included any comorbid diagnoses of neurodegenerative disorders affecting the caudate nucleus such as Huntington’s Disease), non-vascular leukoencephalopathy, or inability to give informed consent.
MRI scans were performed at baseline and at three years. A number of clinical indicators were measured at baseline and at yearly intervals, including measurements of disability, cognition, mood, gait, and medical events such as stroke.
The gait and balance measure utilised for the current study was the Short Physical Performance Battery (SPPB), which tests the ability of subjects to hold themselves in various standing positions (feet in side-by-side, semi-tandem or tandem), measures walking speed over an 8-foot course, and tests ability and speed at rising from a chair [40]. The SPPB is scored out of 15, with a higher score indicating a better performance.
MRI Acquisition
The MRI-protocol consisted of 3D sagittal or coronal T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) images (TE=4–7 ms; TR=10–25 ms; TI=100–950 ms; flip angle=10–30°; voxel size=1×1×1–1.5 mm3; FOV=250mm), axial T2-weighted fast spin echo images (TE=100–120 ms, TR=4000–6000 ms, FOV=250 mm, slice thickness: 5-7.5 mm, interslice gap 0.5 mm) and fluid attenuated inversion recovery (FLAIR) images (TE=100–140 ms, TR=6000–10,000 ms, TI=2000–2400 ms, FOV=250mm, slice thickness 5–7.5mm, interslice gap 0.5mm). The scans were acquired at 1.5 T [preceding description modified from [41]].
Ethical Approval
The LADIS study received ethical approval from local ethics committees in each centre. We received permission from the principal investigators for our analysis of a subset of the MRI scans and associated clinical data, as well as local approval from the Australian National University Human Research Ethics Committee.
Caudate Nucleus and Putamen Tracing
A subset comprising approximately 10% of the sample (n=66) from the LADIS study were selected by the LADIS study group (by GS) from four LADIS centres – Copenhagen, Stockholm (Huddinge), Gothenburg and Helsinki. These centres were chosen in collaboration with one of the authors (GS) and selected due to image quality for tracing and availability. Scans were anonymised by LADIS group researchers before transfer to a MacBook Pro (Apple, Cupertino, CA, USA) computer provided by University of Melbourne based at the Department of Psychological and Addiction Medicine, Australian National University. Subjects with infarcts in the caudate or putamen were excluded.
DICOM (Digital Imaging and Communications in Medicine) files of T1-weighted images were converted to ANALYZE 7.5 format (Mayo BIR, Rochester, NY, USA) using MRIConvert (http://lcni.uoregon.edu/~jolinda/MRIConvert/) to prepare for analysis. Image intensity was standardised by using preset threshold values, based on a pilot analysis of the data, and voxel size was reconstructed as isotropic at 1x1x1mm. The intracranial volume (ICV) was determined in a semi-automated fashion using FSL software (FMRIB Group, Oxford) as a measure to control for brain size. First, brains were skull-stripped with the Brain Extraction Tool (BET) and were then linearly aligned to the MNI152 1mm T1-weighted template. The inverse of the determinant of the affine transformation matrix was multiplied by the ICV of the MNI152 template to produce a measure of ICV for use as a covariate [42].
Caudate nuclei and putamen were manually traced within ANALYZE 10.0b (Mayo BIR, Rochester, NY, USA) through a Region of Interest (ROI) approach on, by one investigator (MM) who was blind to clinical data, using a previously published protocol [43, 44] applied in a number of studies [26, 45-48]. Briefly, this involved tracing images in the axial plane, using the inferior border of the anterior commissure as the inferior boundary (see Figure 2). The resulting binary objects were checked in the sagittal plane, and volumes calculated. Reliability of the tracing was checked by an experienced tracer (JCLL) tracing basal ganglia on a representative sample of the 66 subjects; MM also retraced the representative sample to serve as test-retest reliability. Intraclass correlation coefficients were used to determine reliability of volumetric measurement – intra-rater reliability was 0.944, with inter-rater reliability 0.890. Decisions on whether to proceed to shape analysis were guided by volumetric results: if there was no significant volume change, shape analysis was not attempted. While it is possible to have shape change without volumetric change, and vice versa, our group’s experience is that shape analysis allows localisation of where significant volumetric differences occur. Furthermore, given that volumetric change is very rarely uniform, it follows that overall volume change is driven by a significant localised shape change.
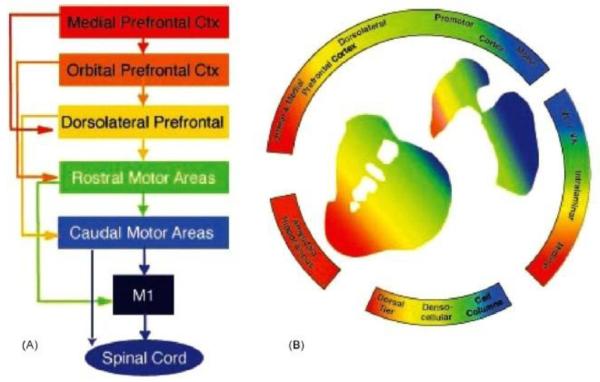
Figure 2.
Diagram demonstrating the functional organisation of A. frontal cortex and B. striatal afferent projections. (A) Schematic illustration of functional connections linking frontal cortical brain regions. (B) Organisation of cortical and subcortical inputs to the striatum. In both (A) and (B), the colours denote functional distinctions. Blue: motor cortex, execution of motor actions; green: premotor cortex, planning of movements; yellow: dorsal and lateral prefrontal cortex, cognitive and executive functions; orange: orbital prefrontal cortex, goal-directed behaviours and motivation; re: medial prefrontal cortex, goal-directed behaviours and emotional processing. Reproduced with permission from Haber, 2003.
Shape Analysis
Shape analysis was undertaken in a semi-automated fashion using the University of North Carolina shape analysis toolkit (http://www.nitrc.org/projects/spharm-pdm/); a detailed description of the methodology is available elsewhere [49, 50]. Segmented 3D binaries are initially processed to ensure interior holes are filled, followed by morphological closing and minimal smoothing. These are then subjected to spherical harmonic shape description (SPHARM), whereby boundary surfaces of each shape are mapped onto the surface of a sphere and the surface coordinates were represented through their spherical harmonic coefficients [51]. The correspondence between surfaces is established by parameter-based rotation, itself based on first-order expansion of the spherical harmonics. The surfaces are uniformly sampled into a set of 1002 surface points and aligned to a study-averaged template for each structure (left and right caudate and putamen) using rigid-body Procrustes alignment [52]. Scaling normalization was performed to remove the effect of head size/intracranial volume, using a surface scaling factor: fi, where fi={Mean(ICV)/ICVi}1/3 [53].
Statistical Analyses
Volumetric data were analysed with paired t-tests to determine change in volume from baseline to 3-year follow-up. ANCOVA was performed to determine whether volume differences were present between severity subgroups as measured by baseline Fazekas score. Relationships between basal ganglia volumes and SPPB scores/walking speeds were assessed with hierarchical regression, with covariates (age, gender and intracranial volume) entered at the first step and the volumes entered at the second step.
With regard to shape analysis, we compute non-parametric statistical tests that compare the local surface coordinates for group mean differences at the 1002 surface locations [49, 50, 53]. A local group difference metric between groups of surface coordinates is derived from the Hotelling T2 two-sample metric [53]. As the shape analysis involves computing 1002 hypothesis tests, one per surface location, a correction for multiple testing is necessary, as an uncorrected analysis would be overly optimistic. The shape analysis uses permutation tests over the Hotelling T2 metric for the computation of the raw uncorrected p-values and uses false discovery rate (FDR) [54] for multiple comparison correction. Correlational analyses were undertaken using Spearman’s rank-correlation co-efficient r, and maps of both r and FDR-corrected p-values are generated.
Results
Demographics, Longitudinal Change and Severity of White Matter Disease
Demographic details of the entire LADIS dataset have been reported [3]. The demographic details and severity of WMC in the LADIS subgroup included in this study were similar to that of the larger dataset. The rates of subsequent transition to disability or death were significantly lower in the study group compared to the rest of the LADIS group, while SPPB scores were not significantly different (Table 1). There was no significant relationship between basal ganglia volumes and the severity of WMC, whether the severity was assessed via the Fazekas scale, total summed WMC volume or Schelten scores (please see online supplementary materials). Longitudinal paired t-tests on baseline and follow-up basal ganglia volumes showed little change in volume over time, apart from some weakly significant increases in caudate volumes in some subsets (<5% of volume, see online supplementary material). I think you mean decreases in caudate volume Table 3 in the appendix reports shirnkage of the right caudate in the mild group and shrinkage of left caudate in the moderate group. This table is a bit confusing as a positive mean difference signifies shirnkage. It might bet better to reverese the sign ie make Mean difference = follow up volume minus baseline volume. Or have I got myself mixed up!!!
Table 1.
Demographic Details
| Variable | Study group n=66 |
Rest of LADIS study (n=573) |
Significance of Difference on t- test/chi-square |
|---|---|---|---|
| Age in years – mean (SD) | 73.2 (4.97) | 74.2 (5.05) | 0.115 (NS) |
| Gender – % male | 51.5 | 44.3 | 0.262 (NS) |
| Mean Caudate Volumes (mm3)* | 2695.4 | N/A | N/A |
| Mean Putamen Volumes (mm3)* | 2195.5 | N/A | N/A |
| Mean Intracranial Volume (cm3) | 1567.9 | N/A | N/A |
| Leukoaraiosis – % mild | 39.7 | 45 | 0.705 (NS) |
| Leukoaraiosis – % mod | 33.8 | 30.5 | 0.705 (NS) |
| Leukoaraiosis – % severe | 26.5 | 24.5 | 0.705 (NS) |
|
% who transitioned to disability or
death at 3 years |
25.8 | 39.6 | 0.035* |
| Average SPPB scores at Baseline | 9.80 | 9.74 | 0.846 (NS) |
|
Average SPPB scores at 3-Year
Follow-Up |
9.69 | 9.11 | 0.119 (NS) |
|
Average Walking Speed at Baseline
(m/sec) |
1.15 | 1.19 | 0.127 (NS) |
|
Average Walking Speed at 3-Year
Follow-up (m/sec) |
1.17 | 1.11 | 0.508 (NS) |
Right plus left volumes
Table 3.
Putamen Volumetry Regression Analyses (Model 2)
| Variable | Time Point | Variable | Standardized Beta |
Sig. | Correlations |
|
|---|---|---|---|---|---|---|
| Partial | Part | |||||
|
SPPB
Score |
Baseline/Study
Entry |
Age | −.259 | .046* | −.268 | −.248 |
|
| ||||||
| Gender | −.288 | .059 | −.254 | −.233 | ||
|
|
||||||
|
Intracranial
Volume |
−.441 | .005 | −.371 | −.355 | ||
|
|
||||||
|
Bilateral Putamen
Volume |
−.084 | .519 | −.088 | −.079 | ||
|
|
||||||
|
3-Year Follow-
up |
Age | −.290 | .045* | −.257 | −.279 | |
|
|
||||||
| Gender | −.097 | .569 | −.068 | −.081 | ||
|
|
||||||
|
Intracranial
Volume |
.077 | .642 | .099 | .066 | ||
|
|
||||||
|
Bilateral Putamen
Volume |
.001 | .993 | .077 | .001 | ||
|
| ||||||
|
Walking
Speed |
Baseline/Study
Entry |
Age | −.400 | .002* | −.371 | −.397 |
|
| ||||||
| Gender | −.271 | .074 | .007 | −.241 | ||
|
|
||||||
|
Intracranial
Volume |
−.328 | .032 | −.216 | −.286 | ||
|
|
||||||
|
Bilateral Putamen
Volume |
.022 | .863 | .008 | .024 | ||
|
|
||||||
|
3-Year Follow-
up |
Age | −.355 | .012* | −.344 | −.350 | |
|
|
||||||
| Gender | −.173 | .295 | .073 | −.149 | ||
|
|
||||||
|
Intracranial
Volume |
−.288 | .077 | −.229 | −.250 | ||
|
|
||||||
|
Bilateral Putamen
Volume |
.008 | .954 | −.011 | .008 | ||
Model 1 = Age, Gender, Intracranial volume Model 2 = Age, Gender, Intracranial volume, Bilateral putamen volume
Volumetry
Initially, volumetric analysis was performed on the manually segmented 3-D objects of caudate and putamen. The results of these analyses guided the shape analysis explorations, based upon our group’s previous experience with shape analysis [24, 46-48]. Regression analysis on these volumes is shown in Table 2. A significant correlation was found between caudate nucleus volumes and both SPPB scores and the walking speed subcomponent of the SPPB at study baseline, but this was not reproduced at three-year follow-up. Intracranial volume was negatively correlated with SPPB at baseline, but not follow-up. There was no significant correlation between putamen volume and measures of gait or balance.
Table 2.
Caudate Nucleus Volumetry Regression Analyses (Model 2)
| Variable | Time Point | Variable | Standardized Beta |
Sig. | Correlations |
|
|---|---|---|---|---|---|---|
| Partial | Part | |||||
|
SPPB
Score |
Baseline/Study
Entry |
Age | −.243 | .040* | −.278 | −.232 |
|
| ||||||
| Gender | −.159 | .261 | −.154 | −.125 | ||
|
|
||||||
|
Intracranial
Volume |
−.370 | .009* | −.350 | −.300 | ||
|
|
||||||
|
Bilateral Caudate
Volume |
.350 | .003* | .390 | .340 | ||
|
|
||||||
|
3-Year Follow-
up |
Age | −.314 | .027* | −.307 | −.302 | |
|
|
||||||
| Gender | −.059 | .721 | −.051 | −.048 | ||
|
|
||||||
|
Intracranial
Volume |
.095 | .555 | .084 | .079 | ||
|
|
||||||
|
Bilateral Caudate
Volume |
.191 | .167 | .194 | .186 | ||
|
| ||||||
|
Walking
Speed |
Baseline/Study
Entry |
Age | −.427 | .001* | −.449 | −.412 |
|
| ||||||
| Gender | −.185 | .194 | −.176 | −.147 | ||
|
|
||||||
|
Intracranial
Volume |
−.274 | .047* | −.266 | −.227 | ||
|
|
||||||
|
Bilateral Caudate
Volume |
.340 | .005* | .373 | .330 | ||
|
|
||||||
|
3-Year Follow-
up |
Age | −.383 | .005* | −.385 | −.368 | |
|
|
||||||
| Gender | −.130 | .412 | −.117 | −.104 | ||
|
|
||||||
|
Intracranial
Volume |
−.266 | .086 | −.243 | −.221 | ||
|
Bilateral Caudate
Volume |
.220 | .096 | .236 | .214 | ||
Model 1 = Age, Gender, Intracranial volume Model 2 = Age, Gender, Intracranial volume, Bilateral caudate volume
Shape Analysis
We applied the shape analysis method to the segmented caudate for the entire dataset. All results were scale-normalised for total intracranial volume. The results presented are based upon FDR corrected p-value maps, together with corresponding local displacement maps. The details of the legend for the analyses are described below the images for ease of reference when reading the images (Figure 4).
Figure 4.
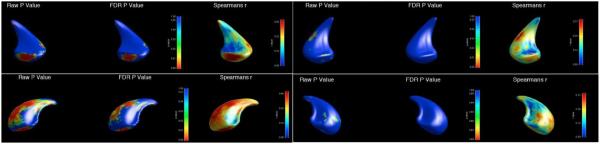
Shape analysis correlating specific areas of the caudate nucleus with performance on the Short Physical Performance Battery (SPPB) at study entry. Clockwise from L top (three images per structure) : 1a,b,c: Left caudate ventral aspect, 2a,b,c: R caudate ventral aspect, 3a,b,c: R dorsal aspect, 4a,b,c: L caudate dorsal aspect. The anterior aspect of the caudate is oriented towards the bottom of the image. Raw P value: unadjusted P value; FDR P value: false discovery rate P value (adjustment for family-wise error); Spearmans r: Spearman’s rank correlation coefficient for SPPB with caudate surface. Scale for images is displayed in the colour bar at the side of the respective images.
Shape deflation was seen unilaterally in the left caudate nucleus, with no significant differences seen in the right caudate. Inward deformation of left caudate shape was found in regions corresponding to the inputs of the dorsolateral prefrontal, premotor and motor cortex, correlating with SPPB (p=0.0017) [32, 55, 56]. Localised shape changes were seen when analysing the walking speed subcomponent of the SPPB, but these did not persist when corrected for FDR.
Discussion
The current study, of older patients with white matter changes, has identified highly significant shape and volume deflation in areas of the left caudate nucleus that subserve motor portions of the fronto-striatal circuits. These results support our hypothesis, and demonstrate patterns similar to those seen in a number of neurodegenerative disorders that manifest structural change in the caudate nucleus such as Huntington’s Disease [57-59], Alzheimer’s Disease [60-63], Progressive Supranuclear Palsy [25, 64], Corticobasal Syndrome and Multiple System Atrophy [65-67]. Accordingly, morphometric (shape and volume) analysis of the caudate may be used as a biomarker which could aid in diagnostic clarity and as an additional marker to track the progression and treatment [68].
Within frontostriatal circuits, the caudate subserves a role in the supplementary motor circuit, connecting to premotor and motor cortex involved in the planning and execution of movements [32]. Impairment of gait and balance was correlated with altered morphology of the caudate. This finding suggests that motor dysfunction is associated with disruption of the supplementary motor frontostriatal circuit. This is borne out by the specific regional atrophy in the postero-lateral aspect of the left caudate. It has been proposed that a purpose of hemispheric lateralisation is to encode constrained repertoires of cognition, emotion and behaviour within the left hemisphere [69]. Thus, the specific atrophy of the left caudate may, in part, reflect the process of loss of the physical substrate of the entrained gait and balance behaviours under frontal control in frontostriatal circuits. A parsimonious explanation for lateralization may be that the corresponding morphologic change was not detected in the right caudate due to sample size.
The lack of correlation between white matter lesion load and striatal volumes, and between caudate morphology and SPPB at follow-up may be partially explained by a survivor effect, i.e. the selection of a group for which MRI were available in 3-year follow-up necessarily resulted in a sample biased to lesser severity of disability in general (as seen in Table 1). Thus a correlation evident at baseline may have much less predictive value and hence correlation at follow-up. This survivor effect may also, in part, be responsible for the lack of atrophy demonstrated in basal ganglia volumes over this period (see online supplementary material) – indeed, there was a statistically significant (although small, <5%) increase in caudate volumes of some subsets, which may reflect some compensation as the patient ages but is more likely to be a statistical artefact. The small but highly significant negative correlation between intracranial volume and SPPB at baseline implies higher ICV is correlated with lower SPPB, signifying poorer motor function. That this is not replicated in the 3-year follow-up may also represent a survivor effect, but is admittedly difficult to explain.
The possible mechanisms of caudate morphology alterations in WMC are admittedly speculative. These mechanisms comprise: possible deafferentation of striatal inputs through direct anatomical disruption by white matter hyperintensities [68], direct vascular damage to the caudate and generalised cerebral atrophy as a result of WMC. We consider the former most likely, and at least in our study, the latter two factors were controlled for by exclusion and as a covariate/scaling respectively.
Limitations of this study include the small sample size. However, as manual tracing is a time-intensive process, the size of this study compares favourably to other shape analysis studies utilising similar methodology [46-48]. Replication of these results, particularly the preponderance of left-sided deflation, will be useful in order to confirm the degree and laterality of shape deflation in individuals with diffuse white-matter hyperintensities. Analysing the rest of the LADIS dataset would be a useful next step, but the large number of scans (600+) involved may require a more automated and less manually intensive segmentation method.
Conclusion
We have demonstrated that the caudate nucleus may be a possible physical substrate and hence, a potential biomarker, for gait and balance in persons with WMC. Recent related research has highlighted that impaired gait, measured as walking speed, is associated with both progression to dementia and increased mortality [33-35]. Thus having identified a possible physical substrate and component of frontostriatal circuits implicated in cortical control of gait and balance is a significant advance. In addition, we have demonstrated a correlation between caudate morphology, responsible for mediation of motor function, and a measure of gait, balance and walking speed. We propose that these methods of shape analysis of the striatum should be applied, with the advance of automated segmentation, to the entire LADIS dataset, and similar datasets to determine if our findings may be replicated, and extended by correlation with automated quantification of white matter hyperintensities. Thus we may derive a reliable neuroimaging biomarker of cognitive and gait dysfunction in cerebrovascular disease, suitable for monitoring disease progression and potentially predictive of dementia and adverse outcomes.
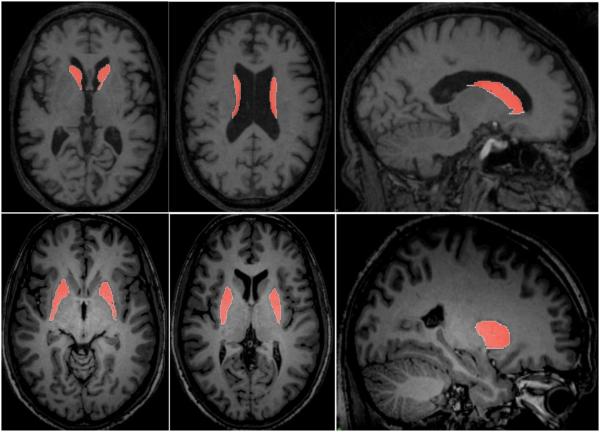
Figure 3.
Representative slices from manual tracing of the caudate nuclei, above, and the putamina, below.
Acknowledgements
A preliminary version of the data analysis for this paper was presented as a poster at the European Federation of Neurological Societies, Stockholm, Sweden, September 2012 and was published as an abstract in the European Journal of Neurology[37]. This study was supported by the European Union (grant QLRT-2000-00446, Impact of age-related brain white matter changes on transition to disability in the elderly ‘Leukoaraiosis and Disability’). The Danish cohort study was supported, in part, by the Danish Velux Foundation. JCLL self-funded travel costs to assist in conduct of this research in Melbourne and Stockholm. This research has made use of the SMILE medical imaging laboratory at Karolinska University Hospital and Karolinska Institute, Stockholm, Sweden. MS was supported by the NIH grant U54 EB005149, National Alliance for Medical Image Computing (NA-MIC).
References
- 1.Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 2.Lindgren A, Roijer A, Rudling O, et al. Cerebral lesions on magnetic resonance imaging, heart disease, and vascular risk factors in subjects without stroke. A population-based study. Stroke. 1994;25:929–934. doi: 10.1161/01.str.25.5.929. [DOI] [PubMed] [Google Scholar]
- 3.Pantoni L, Basile AM, Pracucci G, et al. Impact of age-related cerebral white matter changes on the transition to disability – the LADIS study: Rationale, design and methodology. Neuroepidemiology. 2005;24:51–62. doi: 10.1159/000081050. [DOI] [PubMed] [Google Scholar]
- 4.Group LS. 2001-2011: A decade of LADIS (Leukoaraiosis and disability) study: What have we learned about white matter changes and small vessel disease? Cerebrovascular Diseases. 2011;32:577–588. doi: 10.1159/000334498. [DOI] [PubMed] [Google Scholar]
- 5.Inzitari D, Pracucci G, Poggesi A, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. Bmj. 2009;339:b2477–b2477. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teodorczuk A, Firbank MJ, Pantoni L, et al. Relationship between baseline white-matter changes and development of late-life depressive symptoms: 3-year results from the LADIS study. Psychological Medicine. 2009;40:603. doi: 10.1017/S0033291709990857. [DOI] [PubMed] [Google Scholar]
- 7.Teodorczuk A, O'Brien JT, Firbank MJ, et al. White matter changes and late-life depressive symptoms: Longitudinal study. The British Journal of Psychiatry. 2007;191:212–217. doi: 10.1192/bjp.bp.107.036756. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt R, Ropele S, Ferro J, et al. Diffusion-Weighted Imaging and Cognition in the Leukoariosis and Disability in the Elderly Study. Stroke. 2010;41:e402–e408. doi: 10.1161/STROKEAHA.109.576629. [DOI] [PubMed] [Google Scholar]
- 9.Jokinen H, Kalska H, Ylikoski R, et al. MRI-Defined Subcortical Ischemic Vascular Disease: Baseline Clinical and Neuropsychological Findings. Cerebrovascular Diseases. 2009;27:336–344. doi: 10.1159/000202010. [DOI] [PubMed] [Google Scholar]
- 10.Jokinen H, Gouw AA, Madureira S, et al. Incident lacunes influence cognitive decline: The LADIS study. Neurology. 2011;76:1872–1878. doi: 10.1212/WNL.0b013e31821d752f. [DOI] [PubMed] [Google Scholar]
- 11.Geerlings MI, Appelman APA, Vincken KL, Mali WPTM, van der Graaf Y. Association of White Matter Lesions and Lacunar Infarcts With Executive Functioning: The SMART-MR Study. American Journal of Epidemiology. 2009;170:1147–1155. doi: 10.1093/aje/kwp256. [DOI] [PubMed] [Google Scholar]
- 12.Van der Flier WM, Van Straaten ECW, Barkhof F, et al. Small Vessel Disease and General Cognitive Function in Nondisabled Elderly: The LADIS Study. Stroke. 2005;36:2116–2120. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 13.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: The LADIS Study. Neurology. 2008;70:935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 14.Blahak C, Baezner H, Pantoni L, et al. Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS study. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80:608–613. doi: 10.1136/jnnp.2008.154633. [DOI] [PubMed] [Google Scholar]
- 15.Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. Bmj. 2009;339:b4460–b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Studenski S, Perera S, Patel K, et al. Gait Speed and Survival in Older Adults. Journal of the American Medical Association (JAMA) 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodge HH, Mattek NC, Austin D, Hayes TL, Kaye JA. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology. 2012;78:1946–1952. doi: 10.1212/WNL.0b013e318259e1de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodal, editor. The Central Nervous System: Structure and Function. 3rd Oxford University Press; New York: 2004. [Google Scholar]
- 19.Côté L, Crutcher MD. The basal ganglia. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 3rd Appleton & Lange; Connecticut: 1991. [Google Scholar]
- 20.Nolte, editor. The Human Brain: An Introduction to its Functional Anatomy. 6th Mosby-Elsevier; Philadelphia: 2009. [Google Scholar]
- 21.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. Journal of Neurology, Neurosurgery and Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huppertz H-J, Kröll-Seger J, Danek A, Weber B, Dorn T, Kassubek J. Automatic striatal volumetry allows for identification of patients with chorea-acanthocytosis at single subject level. Journal of Neural Transmission. 2008;115:1393–1400. doi: 10.1007/s00702-008-0094-8. [DOI] [PubMed] [Google Scholar]
- 23.Ghaemi M. Differentiating multiple system atrophy from Parkinson's disease: contribution of striatal and midbrain MRI volumetry and multi-tracer PET imaging. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73:517–523. doi: 10.1136/jnnp.73.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Looi JCL, Lindberg O, Zandbelt BB, et al. Caudate Nucleus Volumes in Frontotemporal Lobar Degeneration: Differential Atrophy in Subtypes. American Journal of Neuroradiology. 2008;29:1537–1543. doi: 10.3174/ajnr.A1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Looi JCL, Macfarlane MD, Walterfang M, et al. Morphometric analysis of subcortical structures in progressive supranuclear palsy: In vivo evidence of neostriatal and mesencephalic atrophy. Psychiatry Research: Neuroimaging. 2011;194:163–175. doi: 10.1016/j.pscychresns.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Looi JCL, Tatham V, Kumar R, et al. Caudate nucleus volumes in stroke and vascular dementia. Psychiatry Research: Neuroimaging. 2009;174:67–75. doi: 10.1016/j.pscychresns.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Alexander GE, DeLong MR, Strick PL. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 28.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. Journal of Neuroscience. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamali A, Kramer LA, Hasan KM. Feasibility of prefonto-caudate pathway tractography using high-resolution diffusion tensor tractography at 3T. Journal of Neuroscience Methods. 2010;191:249–254. doi: 10.1016/j.jneumeth.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehericy S, Ducros M, Van De Moortele P-Fo, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- 31.Di Martino A, Scheres A, Margulies DS, et al. Functional Connectivity of Human Striatum: A Resting State fMRI Study. Cerebral Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 32.Haber S. The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Bruce-Keller A, Brouilette RM, Tudor-Locke C, et al. Assessment of cognition, physical performance, and gait in the context of mild cognitive impairment and dementia. Journal of the American Geriatrics Society. 2012;60:176–177. doi: 10.1111/j.1532-5415.2011.03762.x. [DOI] [PubMed] [Google Scholar]
- 34.Bruce-Keller AJ, Brouillette RM, Tudor-Locke C, et al. Assessment of cognition, physical performance, and gait in the context of mild cognitive impairment and dementia. Journal of the American Geriatric Society (JAGS) 2012;60:176–177. doi: 10.1111/j.1532-5415.2011.03762.x. [DOI] [PubMed] [Google Scholar]
- 35.Gomes de Melo Coelho F, Stella F, Pires de Andrade P, et al. Gait and risk of falls associated with frontal cognitive functions at different stages of Alzheimer's disease. Aging, Neuropsychology and Cognition. 2012 doi: 10.1080/13825585.2012.661398. [DOI] [PubMed] [Google Scholar]
- 36.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5T in Alzheimer's dementia and normal aging. American Journal of Neuroradiology. 1987;8:421–426. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 37.Macfarlane MD, Walterfang M, Looi JCL, et al. Altered caudate morphology in leukoaraiosis is associated with motor dysfunction in a subset of LADIS (leukoaraiosis and disability in the elderly study) European Journal of Neurology. 2012;19:93. [Google Scholar]
- 38.Gouw AA, Flier WM, Straaten ECW, et al. Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. Journal of Neurology. 2006;253:1189–1196. doi: 10.1007/s00415-006-0193-5. [DOI] [PubMed] [Google Scholar]
- 39.Lawton MP, Brody EM. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 40.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. Journal of Gerontology. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 41.Ryberg C, Rostrup E, Paulson OB, et al. Corpus callosum atrophy as a predictor of age-related cognitive and motor impairment: A 3-year follow-up of the LADIS study cohort. Journal of the Neurological Sciences. 2011;307:100–105. doi: 10.1016/j.jns.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 42.ENIGMA Genome-Wide Association Meta-Analysis of Hippocampal Volume via the ENIGMA Consortium; Organisation for Human Brain Mapping Annual Meeting; Quebec City, Quebec. 2011. [Google Scholar]
- 43.Looi J, Lindberg O, Liberg B, et al. Volumetrics of the caudate nucleus: Reliability and validity of a new manual tracing protocol. Psychiatry Research: Neuroimaging. 2008;163:279–288. doi: 10.1016/j.pscychresns.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Looi JCL, Svensson L, Lindberg O, et al. Putaminal Volume in Frontotemporal Lobar Degeneration and Alzheimer Disease: Differential Volumes in Dementia Subtypes and Controls. American Journal of Neuroradiology. 2009;30:1552–1560. doi: 10.3174/ajnr.A1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Looi JCL, Spulber G, Julin P, et al. Caudate nucleus volumes in the Leukoaraioisis and Disability in the Elderly Study – a pilot case-control study of longitudinal change. In: Jacobsen SR, editor. Vascular Dementia: Risk Factors, Diagnosis and Treatment. Nova Science Publishers; New York: 2011. [Google Scholar]
- 46.Looi JCL, Walterfang M, Styner M, et al. Shape analysis of the neostriatum in subtypes of frontotemporal lobar degeneration: Neuroanatomically significant regional morphologic change. Psychiatry Research: Neuroimaging. 2011;191:98–111. doi: 10.1016/j.pscychresns.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Looi JCL, Walterfang M, Styner M, et al. Shape analysis of the neostriatum in frontotemporal lobar degeneration, Alzheimer's disease, and controls. Neuroimage. 2010;51:970–986. doi: 10.1016/j.neuroimage.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Walterfang M, Looi JCL, Styner M, et al. Shape alterations in the striatum in chorea-acanthocytosis. Psychiatry Research: Neuroimaging. 2011;192:29–36. doi: 10.1016/j.pscychresns.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Styner M, Oguz I, Xu S, et al. Framework for the statistical shape analysis of brain structures using SPHARM-PDM. Insight Journal. 2006;1:1–21. [PMC free article] [PubMed] [Google Scholar]
- 50.Levitt JJ, Styner M, Niethammer M, et al. Shape abnormalities of caudate nucleus in schizotypal personality disorder. Schizophrenia Research. 2009;110:127–139. doi: 10.1016/j.schres.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brechbuhler C, Gerig G, Kubler O. Parametrization of closed surfaces for 3-D shape description. Computer Vision, Graphics, Image Processing. 1995;61:154–170. [Google Scholar]
- 52.Bookstein FL. Shape and the information in medical images: a decade of the morphometric synthesis. Computer Vision and Image Understanding. 1997;66:97–118. [Google Scholar]
- 53.Styner M, Oguz I, Xu S, Pantazis D, Gerig G. Statistical group differences in anatomical shape analysis using the Hotelling T2 metric. Proc SPIE 6512, Medical, Imaging. 2007;65123:z65121–z65111. [Google Scholar]
- 54.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 55.Leh S, Ptito A, Chakravarty M, Strafella A. Fronto-striatal connections in the human brain: A probabilistic diffusion tractography study. Neuroscience Letters. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Draganski B, Kherif F, Kloppel S, et al. Evidence for Segregated and Integrative Connectivity Patterns in the Human Basal Ganglia. Journal of Neuroscience. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Douaud G, Gaura V, Ribeiro MJ, et al. Distribution of grey matter atrophy in Huntington’s disease patients: A combined ROI-based and voxel-based morphometric study. NeuroImage. 2006;32:1562–1575. doi: 10.1016/j.neuroimage.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 58.Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 59.Hobbs NZ, Henley SMD, Wild EJ, et al. Automated quantification of caudate atrophy by local registration of serial MRI: Evaluation and application in Huntington's disease. NeuroImage. 2009;47:1659–1665. doi: 10.1016/j.neuroimage.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Barber R. Volumetric MRI study of the caudate nucleus in patients with dementia with Lewy bodies, Alzheimer's disease, and vascular dementia. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72:406–407. doi: 10.1136/jnnp.72.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Jong LW, van der Hiele K, Veer IM, et al. Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study. Brain. 2008;131:3277–3285. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Jong LW, Ferrarini L, van der Grond J, et al. Shape abnormalities of striatum in Alzheimer’s Disease. Journal of Alzheimer's Disease. 2011;23:49–59. doi: 10.3233/JAD-2010-101026. [DOI] [PubMed] [Google Scholar]
- 63.Frederiksen KS, Garde E, Skimminge A, et al. Corpus callosum atrophy in patients with mild Alzheimer's disease. Neurodegenerative Diseases. 2011;8:476–482. doi: 10.1159/000327753. [DOI] [PubMed] [Google Scholar]
- 64.Padovani A. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77:457–463. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boxer AL, Geschwind MD, Belfor N, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Archives of Neurology. 2006;63:81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- 66.Eckert T, Sailer M, Kaufmann J, et al. Differentiation of idiopathic Parkinson's disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. NeuroImage. 2004;21:229–235. doi: 10.1016/j.neuroimage.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 67.Gröschel K, Hauser T-K, Luft A, et al. Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. NeuroImage. 2004;21:714–724. doi: 10.1016/j.neuroimage.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 68.Looi JCL, Walterfang M. Striatal morphology as a biomarker in neurodegenerative disease. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.54. [DOI] [PubMed] [Google Scholar]
- 69.Goldberg E. The New Executive Brain: Frontal Lobes in a Complex World. Oxford University press; New York, NY, USA: 2009. [Google Scholar]