Abstract
Background
Fusarium species are among the most common fungi present in the environment and some species have emerged as major opportunistic fungal infection in human. However, in immunocompromised hosts they can be virulent pathogens and can cause death. The pathogenesis of this infection relies on three factors: colonization, tissue damage, and immunosuppression. A novel Fusarium species is reported for the first time from keratitis in an agriculture worker who acquired the infection from plant material of maize. Maize plants are the natural host of this fungus where it causes stalk rot and seeding malformation under temperate and humid climatic conditions. The clinical manifestation, microbiological morphology, physiological features and molecular data are described.
Methods
Diagnosis was established by using polymerase chain reaction of fungal DNA followed by sequencing portions of translation elongation factor 1 alpha (TEF1 α) and beta-tubulin (BT2) genes. Susceptibility profiles of this fungus were evaluated using CLSI broth microdilution method.
Results
The analyses of these two genes sequences support a novel opportunist with the designation Fusarium temperatum. Phylogenetic analyses showed that the reported clinical isolate was nested within the Fusarium fujikuroi species complex. Antifungal susceptibility testing demonstrated that the fungus had low MICs of micafungin (0.031 μg/ml), posaconazole (0.25 μg/ml) and amphotericin B (0.5 μg/ml).
Conclusion
The present case extends the significance of the genus Fusarium as agents of keratitis and underscores the utility of molecular verification of these emerging fungi in the human host.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-014-0588-y) contains supplementary material, which is available to authorized users.
Keywords: Keratitis, Fusarium temperatum, Maize, Molecular phylogenetics, Infection
Background
Fungal keratitis was first reported by Theodor Leber in 1879 in a farmer who had an eye trauma due to blades used for cutting wheat. Today the infection is known to occur worldwide, particularly in warmer climates, and is caused by a large diversity of fungal species. In temperate regions, fungal keratitis is most commonly caused by Fusarium species[1]. Under (sub) tropical conditions, filamentous fungi are prevalent as causes of infection. Particularly Fusarium and Aspergillus predominate, with up to one-third of cases of traumatic keratitis [2],[3]. On a global scale, fusariosis is one of the most common causes of fungal corneal ulcers [4]-[6].
Keratitis caused by Fusarium is a serious infection and occurs especially among farmers and workers with agricultural occupations. Corneal abrasions occur commonly during harvest, when labor handling decayed and dried plant products is a major risk factor for ocular trauma [7]-[9]. Fungi are one of the possible causes of keratitis and are differentially susceptible to commonly used antifungals. Therefore misdiagnosis potentially leads to visual loss and devastating ocular damage if the infection remains untreated [10].
Fusarium is a genus of more than 200 species of molds that are widely distributed in soil, on terrestrial plants, in plant debris and on other organic substrates. Numerous agents of diseases of plants and cold-blooded animals are known [11], but only a few have been recognized as causing infections in humans [12]. Fusarium infections in immunocompetent hosts are mostly associated with superficial mycosis such as onychomycosis and keratitis; the first reported case of a Fusarium eye infection dates back to 1958 [13]. Recently, deep and systemic infections are observed in immunocompromised patients, with increasing frequency [14].
Here we present an extraordinary case of keratitis in a worker who acquired the infection from plant material of maize. The case is worth reporting not only by its rareness but also its unusual infection in a human. In the present report, this case was initially ascribed to Fusarium oxysporum based on the morphological characters. Since morphological studies are insufficient to determine the correct taxonomic position at the species level in Fusarium, a multilocus DNA sequence study followed by phylogenetic analysis was applied to identify the agent of this case. As a result, Fusarium temperatum is reported as a new causative agent of human keratitis.
Case report
An agriculture worker presented with a corneal ulceration. The disorder had started 12 days earlier when patient suffered from a trauma in the eye with maize plant materials during harvest. Before presenting to the hospital, the patient was seen by a local practitioner who prescribed neomycin, polymyxin B, phenylephrine and dexamethasone eye drops for eight days, supposing that a bacterial infection was concerned. On presentation, visual acuity was 20/50 by using Snellen chart. Slit-lamp examination of the right eye showed a 12 mm white-yellowish central corneal epithelial defect with irregular and raised edges, along with intense hyperemia of the conjunctiva, photophobia and pain (Figure 1A).
Figure 1.
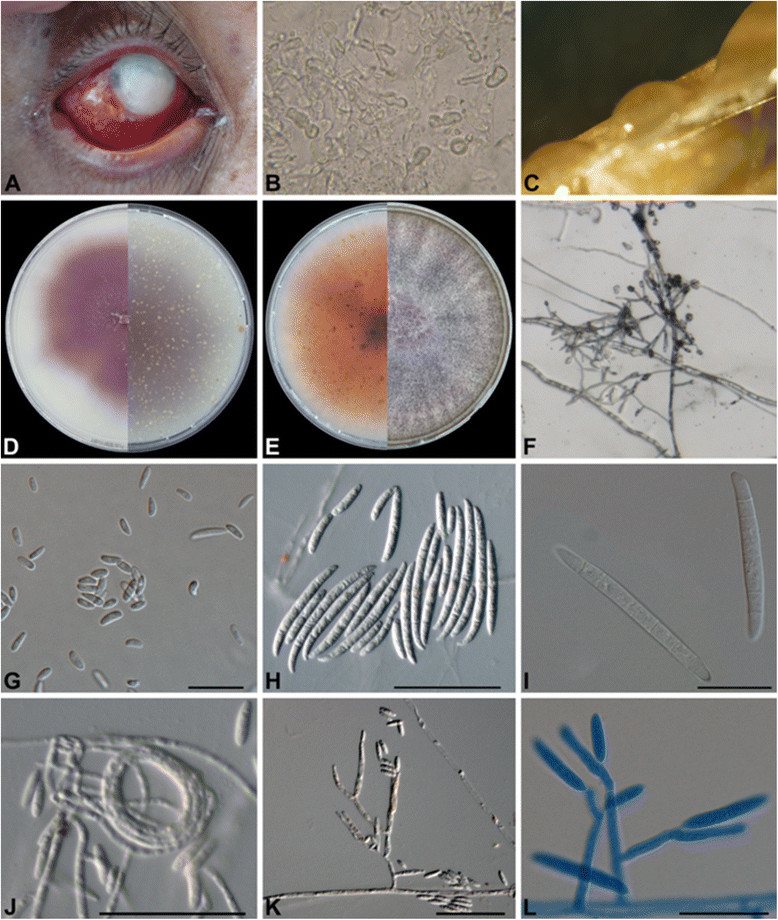
Morphological description of Fusarium temperatum. (A) Slit lamp photograph showing infected cornea involving regions of sclera; (B) KOH mount of the scraping material showing fungal hyaline and nonseptate hyphae (magnification, ×40); (C) Sporodochia present in yellowish orange on CLA; (D) Growth of the isolate F. temperatum on OA, agar pigmentation ranges from colorless to dark purple on; reverse pigmentations in light pink; (E) Growth of isolates on PDA at 25°C; (F) In situ conidiophores with false heads; (G) Microconidia on CLA; (H-I) Macroconida; (J) Coild hyphae; (K-L) Monophialidic and polyphialidic conidiogenous cells. All scale bars, 10 μm.
Corneal scrapings for direct microscopic examination with 10% potassium hydroxide demonstrated multiple irregular, septate, hyaline hyphae (Figure 1A). Samples were inoculated onto Sabouraud’s glucose agar (SGA) plates and incubated for 5 days at 28°C. Fluffy, pink-violet colonies rapidly developed, which microscopically revealed abundant sickle-shaped, septate macroconidia (4–5 μm in length) in addition to microconidia (2–3 μm in length). On the basis of culture and microscopy, the fungus was provisionally identified as F. oxysporum.
Antifungal treatment was initiated with topical natamycin 5% (Laboratorios Grin, Distrito Federal, Mexico) ophthalmic solution, as follows: 2 drops each hour during eight days, then one drop each 4 hours, in addition to itraconazole 200 mg daily. Noteworthy improvement was achieved, and the patient did not return for follow up visits.
Methods
Ethics statement
The study protocol was approved by the Scientific and Ethics Committees of the Hospital General de México (approval number DIC/12/102/3/23) and was performed in accordance with the ethical principles described in the 1964 Declaration of Helsinki. Informed written consent was obtained from the patient prior to their inclusion in the study.
Clinical specimen
Corneal scrapings were collected for microbiological studies from a patient seen in Hospital General de México, O.D, Mexico City, Mexico.
Fungal isolation
Corneal scrapings were inoculated onto Sabouraud’s glucose agar (SGA) plates and incubated for 5 days at 28°C. Subcultures of the causative agent were deposited in the reference collection of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands under accession number CBS 135540, where further identification was undertaken.
Morphology
The fungus grew on culture plates of malt extract ager (MEA; Oxoid, U.K.), oatmeal agar (OA; home-made at CBS), potato dextrose agar (PDA; Oxoid), synthetic nutrient agar (SNA) [15], and carnation leaf agar (CLA) [16]. Culture plates were incubated in the dark for one week at 25°C. Microscopic mounts in lactic acid with cotton blue were made from cultures grown on a PDA plate. Slide cultures were observed after 5 days of incubation at 25°C. Slides were examined and measured with a light microscope (Nikon Eclipse 80i), and pictures were taken using a Nikon digital-sight DS-5 M camera attached to the microscope.
Physiology
Cardinal growth temperatures were determined on PDA with isolates incubated in the dark for one week at 25, 27, 30, 33, 35, 36, 37 and 40°C.
DNA extraction
DNA was extracted following the Quick CTAB protocol. 1–10 mm3 fungal material was transferred to two mL screw-capped tubes filled with 490 μL CTAB-buffer 2 × and 6–10 acid-washed glass beads. 10 μL Proteinase K were added and mixed thoroughly on a MoBio vortex for 10 min. 500 μL Chloroform: isoamylalcohol (24:1) was added and shaken for 2 min after incubation for 60 min at 60°C. Tubes were centrifuged for 10 min at 14,000 r.p.m. The supernatant was collected in a new Eppendorf tube. To ~400 μL DNA sample 2/3 vol (~270 μL) of ice-cold iso-propanol was added and centrifuged again at 14,000 r.p.m. for 10 min and the upper layer was dissolved in 1 mL ice-cold 70% ethanol. Tubes were centrifuged again at 14,000 r.p.m. for 2 min, air-dried and re-suspended in 50 μL TE-buffer. The quality of genomic DNA was verified by running 2–3 μL on a 0.8% agarose gel. DNA was quantified with a NanoDrop 2000 spectrophotometer (Thermo Fisher, Wilmington, U.S.A.). Samples were stored at -20°C until use.
DNA amplification and sequencing
Two gene regions were amplified directly from the genomic DNA for multilocus sequence typing. The primer pairs for the genes were EF1 and EF2 [17], BT-2a [18] and BT-2b [19] (primers listed in Table 1). PCR reaction mixture (12.5 μL final vol) contained 10 × PCR buffer 1.25 μL, water 7.5 μL, dNTP mix (2.5 mm) 0.5 μL, 0.25 μL of each primer (10 pmol), Taq polymerase (5 U/μL) 0.05 μL, DMSO 0.7 μL, and template DNA (100 ng/μL) 1 μL. Amplification was performed in an ABI PRISM 2720 (Applied Biosystems, Foster City, U.S.A.) thermocycler as follows: 95°C for 4 min, followed by 35°Cycles consisting of 95°C for 45 sec, 52°C for 30 sec and 72°C for 2 min, and a delay at 72°C for 7 min. Annealing temperature was changed to 58°C for the BT2 gene. PCR products were visualized by electrophoresis on a 1% (w/v) agarose gel. Amplicons were purified using exoSAP. Both strands of the PCR fragments were sequenced with the above-mentioned primers. The ABI PrismH Big DyeTM Terminator v. 3.0 Ready Reaction Cycle Sequencing Kit (Applied Biosystems) was used for sequencing PCR. Sequences were determined with an ABI PRISM™ 3,100 Genetic Analyzer (Applied Biosystems). Sequencing PCR was performed as follows: 1 min at 95°C, followed by 30°Cycles consisting of 10 sec at 95°C, 5 sec at 50°C and 2 min 60°C. Reactions were purified with Sephadex G-50 fine (GE Healthcare Bio-Sciences, Uppsala, Sweden) and sequencing was done on an ABI 3730XL automatic sequencer (Applied Biosystems) with ABI PRISM BigDyeTM terminator cycle sequencing kit.
Table 1.
PCR primers used for amplification
Phylogenetic analyses
A consensus sequence was computed from the forward and reverse sequences with SeqMan from the Lasergene package (DNAstar, Madison, WI). Thirty eight sequences of species of the Fusarium fujikuroi species complex (FFSC) were included and retrieved from GenBank, including two sequences of the F. oxysporum species complex (FOSC) for TEF1 α and BT2 markers as an outgroup (Additional file 1: Table S1). The sequences were aligned using MAFFT v. 7.127 (http://mafft.cbrc.jp), followed by manual adjustments with MEGA v. 5.2. A combined alignment was constructed for both TEF1 α and BT2 markers. The best-fit model of evolution was determined by ModelTest v. 0.1.1. Bayesian analysis was performed with MrBayes v. 3.1.2. Four MCMC chains were run simultaneously for 1 × 107 generations. Bayesian phylogenetic tree was constructed. Sequences of CBS 135540 were deposited in GenBank under the accession numbers [GenBank:KF956084] for TEF-1α and [GenBank:KF956080] for BT2 (Additional file 1: Table S1).
Antifungal susceptibility
Antifungal susceptibility testing (AFST) of CBS 135540 was performed by the CLSI broth microdilution method, M38-A2 [20]. The antifungals tested were amphotericin B (Sigma, St. Louis, MO), fluconazole (Pfizer, Groton, CT), itraconazole (Janssen Pharmaceutica, Tilburg Netherlands), voriconazole (Pfizer), posaconazole (Merck, Whitehouse Station, NJ), isavuconazole (Basilea Pharmaceutica, Basel, Switzerland), micafungin (Astellas, Ibaraki, Japan), anidulafungin (Pfizer) and natamycin (DSM, Delft, the Netherlands). For the broth microdilution test, RPMI 1640 medium with glutamine without bicarbonate (Sigma) buffered to pH 7 with 0.165 mol/liter 3-N-morpholinepropanesulfonic acid (Sigma) was used. Isolates were grown on PDA for 5 days and incubated at 25°C and for sporulation. Final inoculum was adjusted to a density of 1.0 to 5.0 × 104 hyphal fragments/spores per ml by adjusting an optical density of 0.13 to 0.18 at 530 nm using a spectrophotometer. Drug-free and mold-free controls were included, and microtiter plates were incubated at 35°C for 72 to 96 h. Three reference strains. Paecilomyces variotii ATCC 22319, Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were included as quality controls. The MIC endpoints were read visually, which, for azoles and amphotericin B, were defined as the lowest concentration at which there was 100% inhibition of growth compared with the drug-free control wells. For echinocandins, minimal effective concentrations (MEC) were defined as the lowest concentration of drug that led to the growth of small, rounded, and compact hyphal forms.
Results
Morphology
The clinical isolates grew and sporulated well on PDA, OA, CLA and SNA at 25, 27, 30 and 33°C and growth was apparent within 2 days on all agar plates. Yellowish orange sporodochia were produced on CLA (Figure 1C). Agar pigmentation ranged from colorless to dark purple on OA; pigmentation of colony reverse was in shades of light pink (Figure 1D). Aerial mycelium was cottony, initially white, becoming pinkish white, turning violet in the colony center in a later stage. Subsequently, colonies spread rapidly, filling the culture plate within 1 week (Figure 1E). Conidiophores in the aerial mycelium were erect, branched, terminating in 1–3 phialides (Figure 1F). On SNA with filter paper, colonies were colorless, later changing the color of the filter papers to pale pink. Microconidia were oval, abundant, grouped in masses; hyaline and non-septate (Figure 1G). Macroconidia hyaline, with 3–6 (mostly 4–5) septa, slender, slightly falcate, with a beaked, curved apical cell and a foot-like basal cell with a thin cell wall (Figure 1H, I). Polyphialides and monophialides were observed (Figure 1K, L). Chlamydospores were not found over 10 days of incubation.
Physiology
Cardinal growth temperatures tests showed optimal development at 25 − 27°C (Figure 2), with a maximum growth temperature at 36°C. No growth was observed at 37 and 40°C. 37°C proved to be fungistatic, but regrowth was observed after incubation at 25°C. Colonies on PDA attained a diameter of approximately 65 mm, and those on OA covered the entire agar surface after 5 days at 25°C. Colonies attained a diameter of about 68 mm at 27°C in the dark on PDA. Colonies on PDA plates incubated at 33°C showed slow growth and attained a diameter of about 32 mm after 5 days.
Figure 2.
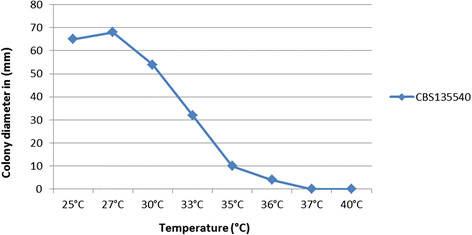
Average growth of Fusarium temperatum . Colony diameters (mm) at different temperatures ranging from 25°C to 40°C, measured after 5 days of incubation on 2% MEA, were calculated for F. temperatum, CBS135540.
Phylogeny
BT2 and TEF-1α partial genes (NCBI JX987074.1 for TEF-1α and KC964140.1 for BT2) were used for identification of clinical isolate CBS 135540. Both genes possessed enough polymorphism, and therefore, were excellent markers with 99–100% accuracy for the identification of Fusarium species to be Fusarium temperatum within the Fusarium fujikuroi species complex. No data were available in the Fusarium MLST database (http://www.cbs.knaw.nl/fusarium) for this isolate.
In order to establish the phylogenetic position of the F. temperatum clade, a general tree was made with MrBayes v. 3.1.2 on the Cipres Portal based on the BT2 (500 bp) and TEF-1α (600 bp) regions. Fifteen species within the Fusarium fujikuroi species complex clade were selected for phylogenetic analyses and sequences of the BT2 and TEF-1α genes were aligned among the sequences available from GenBank (Additional file 1: Table S1). Bayesian analysis was done by using Metropolis-coupled Markov chain Monte Carlo sampling approach to calculate posterior probabilities. Four simultaneous Markov chains, three heated and one cold, were run under a mixed model of sequence evolution and gamma approximation for rate variation among sites. Chains were analysed with random starting trees for 107 generations, sampling from trees every 1000th generation. The burn-in period was set at 25%. Topologies of the trees generated with either gene (TEF-1α and BT2) were concordant. Partition Homogeneity Test (PHT = 0.97) did not detect conflict between loci, and therefore, these two genes were combined to investigate species delimitation using PSR (Figure 3). The BT2 and TEF-1α phylogenetic analyses showed that the reported clinical isolate was nested within the Fusarium fujikuroi species complex and was found to be identical to four environmental strains of F. temperatum.
Figure 3.
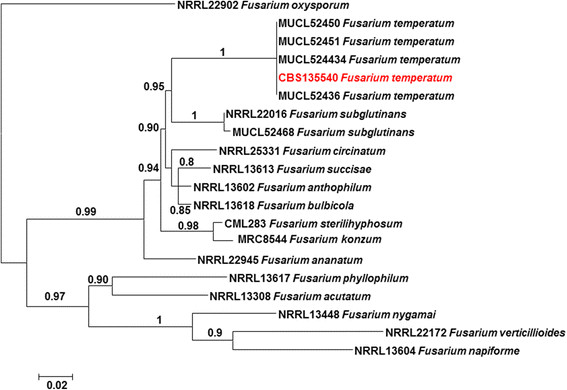
Phylogenetic analysis of Fusarium temperatum. Phylogenetic tree resulting from Bayesian analysis for the TEF-1α and β-tubulin genes (values of 0.8 for Bayesian probability are shown). Fusarium oxysporum (NRRL 22902) was used as the outgroup.
Antifungal susceptibility
Susceptibility testing was performed according to the guidelines of the Clinical and Laboratory Standards Institute document M38-A2. Our strain was highly susceptible to micafungin with an MIC of 0.031 μg/ml, followed by posaconazole with an MIC of 0.25 μg/ml and amphotericin B with an MIC of 0.5 μg/ml. Low MICs were also found for voriconazole (1 μg/ml) followed by isavuconazole, natamycin and anidulafungin, with MICs of 4 μg/ml. The azoles for which high MICs were found were fluconazole (>64 μg/ml) and itraconazole (>16 μg/ml) (Table 2).
Table 2.
MIC values of clinical isolate Fusarium temperatum , CBS135540
| Drug | AMB | FLC | ITC | VOR | POS | NATA | ISA | ANI | MICA |
|---|---|---|---|---|---|---|---|---|---|
| MIC values(μg/ml) | 0.5 | >64 | >16 | 1 | 0.25 | 4 | 4 | 4 | 0.031 |
AMB Amphotericin B, FLC Fluconazole, ITC Itraconazole, VOR Voriconazole, POS Posaconazole, ISA Isavuconazole, NATA Natamycin, ANI Anidulafungin, MICA micafungin.
Discussion
Mycotic keratitis is an important ophthalmologic problem with slow progression that must be distinguished from its bacterial counterpart [21] in view of appropriate treatment. Despite developments in diagnostics and therapy, the infection remains a significant public health problem, occasionally leading to significant visual disability [22]. Fusarium species are among the most common etiologic agents of the disorder [23]-[25] and are problematic because of their therapy-refractive nature. In direct microscopy Fusarium species are indistinguishable from Aspergillus because both produce hyaline, septate hyphae (Figure 1B), and therefore supplementary diagnostics are necessary.
For accurate identification of the causative agent, multi-locus analysis involving of parts of TEF-1α and BT2 genes, known to be informative at the species level in Fusarium[19],[26]-[28] was performed. Identification of the etiological agent as F. temperatum was unambiguous. The present case extends the significance of genus of Fusarium as agents of keratitis and underscores the utility of molecular methods in verification of these emerging fungi in the human host [29].
Fusarium species are distributed worldwide in a wide diversity of habitats such as soil, plant debris, and as pathogens on a wide diversity of plant hosts [30]. Some species synthesize mycotoxins, which may accumulate in infected plant tissue before harvest or in stored agricultural products where they can be harmful for humans [31]. Fusarium in agricultural products should not only be considered as a food spoilers, but also are a risk factor for farmers and harvesters dealing with infected farming material [32], causing traumatic infections.
A remarkable feature in Fusarium is the apparent combination of plant pathogenicity and the ability to cause infections in humans. A study of members of the Fusarium solani species complex demonstrated that strains from clinical specimens, sewage, and plants were all capable of infecting zucchini and growing at 37°C [33]. Zhang et al. [34] noted that a group of clinical isolates were identical to F. solani f. sp. cucurbitae race 2, which infects squashes [35]. Conversely, F. oxysporum f. sp. lycopersici race 2 killing tomato plants was also able to cause disseminated, fatal infection in a murine model [36]. Several studies reported Fusarium subglutinans as an important pathogen of maize, causing stalk and seeding malformation [36],[37], but the species has been reported as a human opportunist [38],[39].
Scauflaire et al. [40] examined the taxonomic status of 30 Fusarium strains isolated from maize fields in Belgium including three isolates named F. subglutinans and described them as a new species, F. temperatum[40]. Subsequently the authors [41] used different pathogenicity tests such as toothpick inoculation to obtain better understanding of the infection process in maize plants and concluded that F. temperatum is a host-specific plant pathogen. The species causes seedling blight chlorosis and forms necrotic lesions in maize stalks; in addition, the species produces mycotoxins. Pintos et al. [42] reported F. temperatum seedling malformations, chlorosis, shoot reduction and stalk rot in maize growing in inoculated soil.
The present case concerns a further example of a plant pathogen in addition to 70 other Fusarium species causing human infection. Such examples are expected to be rare, because in general degradation of plant versus animal components requires entirely different enzymatic machinery. Van Baarlen et al. [43] noted molecular similarity between hypothetical virulence factors in plant and human pathogens, but in practice such species are extremely uncommon [44].
Our patient developed a mycotic keratitis after traumatic introduction with maize leaves during harvest. Its clinical diagnosis was made by the ophthalmologist. At the clinical laboratory, it was first misdiagnosed as F. oxysporum using morphology. These results indicate that morphologic identification has some limitations.
Cardinal growth temperatures showed optimal development at 27°C (Figure 2), with a maximum growth temperature at 36°C. No growth was observed at 37°C and 40°C; these temperatures proved to be fungistatic as regrowth was observed after incubation at 25°C. The relatively low maximum growth temperature allows superficial infections such as keratitis. Our patient’s infection was first clinically diagnosed as a reactional or bacterial ophthalmitis, leading to the use of a preparation that contained two antibiotics, a decongestant (vasopressor), and a synthetic glucocorticoid. It is noteworthy to record that most cases of keratitis are treated on the basis of clinical features with antibiotics and steroids, which are compounds that stimulate fungal growth [45], while co-inoculation of a fungal agent is common. At observation of fungal hyphae in tissue, therapy was changed to natamycin and itraconazole.
The high susceptibility of CBS 135540 was unexpected because of the intrinsic resistance against antifungal drugs in Fusarium in general [46]-[48]. The F. temperatum isolates had low MICs for micafungin (0.031 μg/ml), posaconazole (0.25 μg/ml) and amphotericin B (0.5 μg/ml). Antifungal susceptibility testing proved that itraconazole and fluconazole were ineffective, and therefore clinical improvement of our patient was probably entirely due to natamycin because the present study shows that the natamycin had good activity against F. temperatum (4 μg/ml). Patient was not available for follow-up. In similar cases of keratitis, natamycin, anidulafungin, micafungin, voriconazole and amphotericin B were found to be effective [49].
Conclusion
Fusarium species in general show high degrees of resistance to most antifungals. Using molecular identification to identify a number of rare medically important fungal genera and species, including Fusarium, is very important to predict therapeutic outcome and the emergence of these Fungi. Moreover, this paper provides evidence indicating Fusarium temperatum as a potential human opportunist that may have decreased susceptibility to azoles and other antifungal agents.
Availability of supporting data
The data sets supporting the results of this article are available in the [LabArchives, LLC] repository, [http://dx.doi.org/10.6070/H46D5QZK].
The phylogenetic tree supporting the results of this article is available in the [TreeBASE] repository, [http://purl.org/phylo/treebase/phylows/study/TB2:S16415?x-access-code=ac517f778e8736faf6a6a3e9e4f1b263&format=html].
Authors’ contributions
LVM collected the clinical data and patient’s history. KGC collected the clinical specimen and the clinical data. AB performed the phenotypic identification and helped with the draft of the manuscript. JFM performed the susceptibility test and helped with the draft of the manuscript. AMS performed molecular identification, phenotypic description and wrote the manuscript. ADvD helped with the draft of the manuscript. SdH supervised the coordination and concept of the manuscript. All authors have read and approved the manuscript.
Additional file
Electronic supplementary material
Additional file 1: Table S1.: Strains features used for phylogeny. Collection, origin, and GenBank accession numbers used in the present study for phylogenetic analysis of Fusarium temperatum, CBS 135540. (DOCX 15 KB)
Below are the links to the authors’ original submitted files for images.
Acknowledgment
We thank Kasper Luijsterburg for providing the photographic plates. The work of Abdullah Mohammed Said Al-Hatmi was financially supported by Ministry of Health, Muscat, Oman.
Abbreviations
- TEF1 α
Translation elongation factor 1 alpha
- BT2
Beta-tubulin
- MLST
Multilocus sequence typing
- MEA
Malt extract agar
- OA
Oatmeal agar
- PDA
Potato dextrose agar
- SNA
Synthetic nutrient agar
- CLA
Carnation leaf agar
- PCR
Polymerase chain reaction
- MIC
Minimum inhibitory concentration
- AMB
Amphotericin B
- FLC
Fluconazole
- ITC
itraconazole
- VOR
Voriconazole
- POS
Posaconazole
- NATA
Natamycin
- ISA
Isavuconazole
- ANI
Anidulafungin
- MICA
Micafungin
- MEC
Minimal effective concentrations
Footnotes
Competing interests
The authors declare that they have no competing interest.
Contributor Information
Abdullah M S Al-Hatmi, Email: a.alhatmi@cbs.knaw.nl.
Alexandro Bonifaz, Email: a_bonifaz@yahoo.com.mx.
G Sybren de Hoog, Email: s.hoog@cbs.knaw.nl.
Leticia Vazquez-Maya, Email: letivaz@yahoo.com.mx.
Karla Garcia-Carmona, Email: karlapaola10@gmail.com.
Jacques F Meis, Email: j.meis@cwz.nl.
Anne D van Diepeningen, Email: a.diepeningen@cbs.knaw.nl.
References
- 1.Liesegang TJ, Forster RF. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90:38–47. doi: 10.1016/S0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim MM, Vanini R, Ibrahim FM, Fioriti LS, Furlan EM, Provinzano LM, De Castro RS, de Faria E, Sousa SJ, Melani Rocha E. Epidemiologic aspects and clinical outcome of fungal keratitis in southeastern Brazil. Eur J Ophthalmol. 2009;19:355–361. doi: 10.1177/112067210901900305. [DOI] [PubMed] [Google Scholar]
- 3.Galarreta DJ, Tuft SJ, Ramsay A, Dart JK. Fungal keratitis in London: microbiological and clinical evaluation. Cornea. 2007;26:1082–1086. doi: 10.1097/ICO.0b013e318142bff3. [DOI] [PubMed] [Google Scholar]
- 4.D°Czi I, Gyetvai T, Kredics L, Nagy E. Involvement of Fusariumspp. in fungal keratitis. Clin Microbiol Infect. 2004;10:773–776. doi: 10.1111/j.1469-0691.2004.00909.x. [DOI] [PubMed] [Google Scholar]
- 5.Guarro J, Gene J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:741–754. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- 6.Bernal MD, Acharya NR, Lietman TM, Strauss EC, McLeod SD, Hwang DG. Outbreak of Fusariumkeratitis in soft contact lens wearers in San Francisco. Arch Ophthalmol. 2006;124:1051–1053. doi: 10.1001/archopht.124.7.ecr60006. [DOI] [PubMed] [Google Scholar]
- 7.Chander J, Sharma A. Prevalence of fungal corneal ulcers in northern India. Infection. 1994;22:207–209. doi: 10.1007/BF01716706. [DOI] [PubMed] [Google Scholar]
- 8.Polack FM, Kaufman HE, Newmark E. Keratomycosis. Medical and surgical treatment. Arch Ophthalmol. 1971;85:410–416. doi: 10.1001/archopht.1971.00990050412003. [DOI] [PubMed] [Google Scholar]
- 9.Homa M, Shobana CS, Singh YR, Manikandan P, Selvam KP, Kredics L, Narendran V, Vágvölgyi C, Galg°Czy L. Fusarium keratitis in South India: causative agents, their antifungal susceptibilities and a rapid identification method for the Fusarium solanispecies complex. Mycoses. 2013;56:501–11. doi: 10.1111/myc.12062. [DOI] [PubMed] [Google Scholar]
- 10.Kebabc N, van Diepeningen AD, Ener B, Ersal T, Meijer M, Al-Hatmi AMS, Özkocaman V, Ursavaş A, Çetinoğlu ED, Akaln H. Fatal breakthrough infection with Fusarium andiyazi: new multi-resistant aetiological agent cross-reacting with Aspergillus galactomannan enzyme immunoassay. Mycoses. 2014;57:249–255. doi: 10.1111/myc.12142. [DOI] [PubMed] [Google Scholar]
- 11.Jain PK, Gupta VK, Misra AK, Gaur R, Bajpai V, Issar S. Current status of Fusariuminfection in human and animal. Asian J Anim Veter Adv. 2011;6:201–227. doi: 10.3923/ajava.2011.201.227. [DOI] [Google Scholar]
- 12.Nucci M, Anaissie E. Fusariuminfections in immunocompromised patients. Clin Microbiol Rev. 2007;20:695–704. doi: 10.1128/CMR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikami R, Stemmermann GN. Keratomycosis caused by Fusarium oxysporum. Am J Clin Pathol. 1958;29:257–262. doi: 10.1093/ajcp/29.3.257. [DOI] [PubMed] [Google Scholar]
- 14.Gupta AK, Baran R, Summerbell RC. Fusariuminfections of the skin. Curr Opin Infect Dis. 2000;13:121–128. doi: 10.1097/00001432-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Nirenberg HI. Untersuchungen uber die morphologische und biologische differenzierung in der FusariumSektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft (Berlin-Dahlem) 1976;169:1–117. [Google Scholar]
- 16.Leslie JF, Summerell BA. The Fusarium Laboratory Manual. Ames, Oxford: Blackwell Publishing Ltd; 2006. [Google Scholar]
- 17.O’Donnell K, Sutton DA, Rinaldi MG, Sarver BA, Balajee SA, Schroers HJ, Summerbell RC, Robert VA, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee YH, Kang S, Park B, Geiser DM. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol. 2010;48:3708–3718. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glass NL, Donaldson G. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Appl Environm Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donnel K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusariumare nonorthologous. Mol Phylogenet Evol. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- 20.Reference Method for Broth Dilution Antifungal Susceptibility Testing for Filamentous Fungi; Approved Standard. Document M38-A2. 2008, Clinical and Laboratory Standards Institute, Wayne, PA
- 21.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15:321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Guarro J. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur J Clin Microbiol Infect Dis. 2013;32:1491–1500. doi: 10.1007/s10096-013-1924-7. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhary A, Singh K. Spectrum of fungal keratitis in North India. Cornea. 2005;24:8–15. doi: 10.1097/01.ico.0000126435.25751.20. [DOI] [PubMed] [Google Scholar]
- 24.Punia RS, Kundu R, Chander J, Arya SK, Handa U, Mohan H. Spectrum of fungal keratitis: clinicopathologic study of 44 cases. Int J Opthalmol. 2014;7:114–117. doi: 10.3980/j.issn.2222-3959.2014.01.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Short DP, O’Donnell K, Thrane U, Nielsen KF, Zhang N, Juba JH, Geiser DM. Phylogenetic relationships among members of the fusarium solani species complex in human infections and the descriptions of F. Keratoplasticum sp. nov. and F. petroliphilumstat. nov. Fungal Genet Biol. 2013;53:59–70. doi: 10.1016/j.fgb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Knogge W. Fungal infection of plants. The Plant Cell. 1996;8:1711–1722. doi: 10.1105/tpc.8.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiser D, Jimenez-Gasco M, Kang S, Veeraraghavan I, Ward T, Zhang N, Kuldau G, O’Donnell K. FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Pathol. 2004;110:473–479. doi: 10.1023/B:EJPP.0000032386.75915.a0. [DOI] [Google Scholar]
- 28.O’Donnell K, Nirenberg H, Takayuki A, Cigelnik E. A multigene phylogeny of the Gibberella fujikuroispecies complex: detection of additional phylogenetically distinct species. Mycoscience. 2000;41:61–78. doi: 10.1007/BF02464387. [DOI] [Google Scholar]
- 29.Guarro J, Gene J, Stchigel A. Developments in fungal taxonomy. Clin Microbiol Rev. 1999;12:454–500. doi: 10.1128/cmr.12.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SN. An overview of ecological and habitat aspects in the genus Fusariumwith special emphasis on the soil-borne pathogenic forms. Plant Pathol Bull. 2007;16:97–120. [Google Scholar]
- 31.Coulumbe RA. Biological action of mycotoxins. J Dairy Sci. 1993;76:880–891. doi: 10.3168/jds.S0022-0302(93)77414-7. [DOI] [PubMed] [Google Scholar]
- 32.Tabatabaee A, Mohajernezhadfard Z, Daneshgar F, Mansouri M. Keratomycosis after incidental spillage of vegetative material into the eye: report of two cases. Oman J Ophthalmol. 2013;6:122–126. doi: 10.4103/0974-620X.116659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehl HL, Epstein L. Fusarium soloni species complex isolates conspecific with Fusarium solani f. sp. cucurbitaerace 2 from naturally infected human and plant tissue and environmental sources are equally virulent on plants, grow at 37°C and are interfertile. Environ Microbiol. 2007;9:2189–2199. doi: 10.1111/j.1462-2920.2007.01333.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, O’Donnell K, Sutton DA, Nalim FA, Summerbell RC, Padhye AA, Geiser DM. Members of the Fusarium solanispecies complex that cause infection in both humans and plants are common in the environment. J Clin Microbiol. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortoneda M, Guarro J, Madrid MP, Caracuel Z, Roncero M, Mayayo E, Pietro A. Fusarium oxysporumas a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect Immun. 2004;72:1760–1766. doi: 10.1128/IAI.72.3.1760-1766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lew HJ, Chelkowski J, Pronczuk P, Edinger W. Occurrence of the mycotoxin moniliformin in maize (Zea mays L.) ears infected by Fusarium subglutinans. Food Addit Contam. 1996;13:321–324. doi: 10.1080/02652039609374414. [DOI] [PubMed] [Google Scholar]
- 37.Edwards ET. Studies on the Gibberella fujikuroi var. subglutinans the hitherto undescribed ascigerous stage of Fusarium moniliforme var. subglutinansand its pathogenicity on maize in New South Wales. Dep Agric New South Wales Sci Bull. 1935;49:1–68. [Google Scholar]
- 38.Marasas WFO, Rheeder JP, Lamprecht SC, Zeller KA, Leslie JF. Fusarium andiyazisp.nov., a new species from sorghum. Mycologia. 2001;93:1203–1210. doi: 10.2307/3761681. [DOI] [Google Scholar]
- 39.Britz H, Coutinho TA, Wingfield MJ, Marasas WFO, Gordon TR, Leslie JF. Fusarium subglutinans f. sp. pini represents a distinct mating population in the Giberrella fujikuroispecies complex. Appl Environ Microbiol. 1999;65:1198–1201. doi: 10.1128/aem.65.3.1198-1201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scauflaire J, Gourgue M, Munaut F. Fusarium temperatum sp. nov. from maize, an emergent species closely related to Fusarium subglutinans. Mycologia. 2011;103:586–597. doi: 10.3852/10-135. [DOI] [PubMed] [Google Scholar]
- 41.Scauflaire J, Gourgue M, Callebaut A, Munant F. Fusarium temperatum, a mycotoxin-producing pathogen of maize. Eur J Plant Pathol. 2012;133:911–922. doi: 10.1007/s10658-012-9958-8. [DOI] [Google Scholar]
- 42.Pintos V, Aguín C, Chaves P, Ferreiroa-Martínez V, Sainz M, Scauflaire J, Munaut F, Mansilla V. First report of fusarium temperatumcausing seedling blight and stalk Rot on maize in Spain. Plant Dis. 2013;97:1252–1252. doi: 10.1094/PDIS-02-13-0167-PDN. [DOI] [PubMed] [Google Scholar]
- 43.Van Baarlen P, Van Belkum A, Summerbell R, Crous PW, Thomma B. Molecular mechanisms of pathogenicity: how do pathogenic microorganisms develop cross-kingdom host jumps? FEMS Microbiol Rev. 2007;31:239–277. doi: 10.1111/j.1574-6976.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- 44.De Hoog G, Guarro J, Gene J, Figueras MJ. Atlas of Clinical Fungi. Utrecht, the Netherlands: Centraalbureau voor Schimmelcultures; 2000. [Google Scholar]
- 45.Vanzzini V, Manzano-Gayosso P, Hernández-Hernández F, Méndez-Tovar LJ, Gómez-Leal A, López R. Mycotic keratitis in an eye care hospital in Mexico City. Rev Iberoam Micol. 2010;27:57–61. doi: 10.1016/j.riam.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Lewis R, Wiederhold N, Klepser M. In vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against aspergillus, fusarium, and scedosporiumspp. Antimicrob Agents Chemother. 2005;49:945–995. doi: 10.1128/AAC.49.3.945-951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paphitou N, Ostrosky-Zeichner L, Paetznick V, Rodríguez J, Chen E, Rex J. In vitro activities of investigational triazoles against Fusariumspecies: effects of inoculum size and incubation time on broth microdilution susceptibility test results. Antimicrob Agents Chemother. 2002;46:3298–3300. doi: 10.1128/AAC.46.10.3298-3300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pujol I, Guarro J, Gené J, Sala J. In-vitro antifungal susceptibility of clinical and environmental Fusariumspp. strains. J Antimicrob Chemother. 1997;39:163–167. doi: 10.1093/jac/39.2.163. [DOI] [PubMed] [Google Scholar]
- 49.Oechsler R, Yamanaka T, Paulo Bispo J, Sartori J, Yu MC, Melo A, Miller D, Hofling-Lima A. Fusarium keratitis in Brazil: genotyping, in vitrosusceptibilities, and clinical outcomes. Clin Ophthalmol. 2013;7:1693–1701. doi: 10.2147/OPTH.S40063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1.: Strains features used for phylogeny. Collection, origin, and GenBank accession numbers used in the present study for phylogenetic analysis of Fusarium temperatum, CBS 135540. (DOCX 15 KB)


