Figure 1.
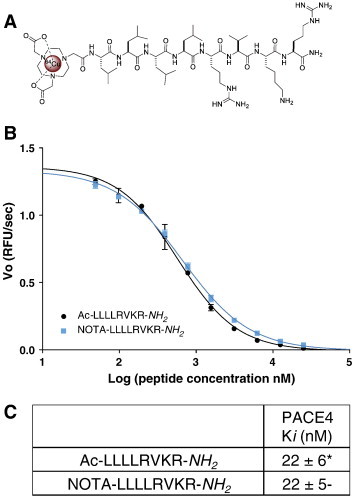
NOTA-LLLLRVKR-NH2 in vitro affinity toward PACE4. (A) Structure of the 64Cu-NOTA-LLLLRVKR-NH2. (B) PACE4 competitive enzyme kinetic assay with the cleavable fluorogenic substrate; Pyr-Arg-Thr-Lys-Arg-methyl-coumaryl-7-amide using increasing doses of both NOTA-ML and Ac-ML inhibitors. The presented plot is a representative experiment where Vo is the velocity of reaction in RFU/s. (B) Peptide Ki against recombinant PACE4. Data are mean ± SD (n = 3). *Data from Levesque et al. [22].
