Abstract
Background:
There are conflicting reports regarding the association between coronary artery disease (CAD) and mild cognitive impairment (MCI). Volumetric Magnetic resonance imaging (MRI) investigations have been considered as an objective biomarker for MCI. In this study, we determined the relationship between the regional brain volumes and the extent of CAD in MCI patients and cognitively normal controls.
Materials and Methods:
In a case-control study a subset of MCI patients (n = 20) and cognitively normal controls (n = 20), aged 66.4 ± 4.6 and 65.3 ± 3.9 respectively, from subjects who were recently admitted to cardiac catheterization facilities in two general hospitals were selected. All subjects underwent a clinical interview, biochemical measures, neuropsychological testing and Neuropsychiatry Unit COGnitive assessment tool. Video records of coronary angiography were scored with the Gensini method. For volumetric evaluation of regions of interest, brain MRI scans was processed using the FreeSurfer software package the relationship between the regional brain volumes and the extent of CAD in MCI patients and cognitively normal controls were compared.
Results:
We have found that, there were significant differences between the two groups in volumes of left fusiform (P = 0.039), left pars triangularis (P = 0.003) and left superior temporal gyrus (P = 0.009), after controlling for intracranial volumes. Higher Gensini scores were associated with reduced volumes of total cortical volume (P = 0.047, R = −0.4), left precuneus (P = 0.022, R = −0.5), right inferior parietal lobule (P = 0.011, R = −0.5) and left supra marginal gyrus (P = 0.035, R = −0.04) in MCI.
Conclusion:
In MCI, a greater degree of coronary stenosis correlates with greater loss of gray matter in specific brain regions relevant to cognitive function. This, however, was not the case for cognitively normal subjects.
Keywords: Angiography, brain volumes, coronary artery disease, magnetic resonance imaging, mild cognitive impairment
INTRODUCTION
Osteoarthritis, osteoporosis, cardiovascular disease, dementia, metabolic syndromes (hypertension, diabetes) and impaired hearing and vision are among the most common problems in the elderly population.
Dementia and cardiovascular disease are associated with a high degree of community burden due to the need for the long-term care with specific social services and considerable cost.[1,2,3] It is estimated that dementia affects 4.6 million new patients every year, and this number will double every 20 years to reach 81.1 million by 2040.[4] According to the global burden of disease (GBD) estimations of 2003 World Health Report, dementia has contributed to 11.2% of the years lived with disability (YLD) in people aged 60 years and older.[1]
Mild cognitive impairment (MCI) is a transitional state between normal aging and dementia characterized by cognitive decline, without significant impairment in activities of daily living.[5,6] MCI affects 12-18% of individuals aged 65 and over.[7] People with MCI develop dementia at the annual rate of 5-16%, while this is 1-2% for healthy elderly individuals.[8] MCI is classified into two types, amnestic MCI (a-MCI) and non-amnestic MCI (na-MCI). Patients with a-MCI have predominant memory deficit, while na-MCI is characterized by deficits in cognitive domains other than memory (mainly in executive functioning).[5,9] Increased age, lower education, late life depression,[10] ApoliporoteinE4 polymorphism[9,11] and vascular disease[12,13] have been reported as risk factors for MCI. Meanwhile, The identification of MCI risk factors is an important factor for more effective prevention, treatment and rehabilitation.[14]
The burden of coronary disease has also shifted toward elderly persons in recent decades.[15] According to the GBD report the YLD rate was 5% for cardiovascular disease.[1] While the existence of a link between atherosclerosis and dementia is well known,[16,17,18,19,20,21] there are conflicting reports regarding the association between coronary artery disease (CAD) and MCI. Studies on patients with CAD, who were scheduled for coronary angiography, percutaneous cardiac intervention or cardiac surgery revealed lower performances on all cognitive tests in comparison with healthy controls.[22,23,24] In one study, 35-45% of patients undergoing selective coronary artery bypass grafting, were found to have MCI.[22] Siuda et al. reported that MCI patients with vascular risk factors showed more intensive cognitive dysfunction.[14] Zulli et al. revealed that there was an increased prevalence of silent myocardial infarction in MCI patients.[25] In a population-based study of 1969 subjects of aged 70-89 years, there was a positive association between the presence of stenosis in coronary angiography and na-MCI.[26] Postmortem studies in CAD patients with no clinical dementia have identified atrophy, degenerative changes, and amyloid plaques in the hippocampus and cortical areas.[26,27,28,29] These findings pointed to an active degenerative process in the brain of patients with CAD, which suggests they be at risk for a future diagnosed of MCI or dementia.
Biomarkers for the investigation and early identification of MCI include chemical, genetic or neuroimaging investigations.[29,30,31] Cross-sectional studies of volumetric magnetic resonance imaging (MRI) investigations using manual tracing methods have identified atrophy of medial temporal lobe structures, including the hippocampus and entorhinal cortex (ERC).[32,33,34,35] The most common findings in automated volumetric MRI investigations have been reduced volume in the hippocampus, amygdala, ERC, parahippocampal gyrus, and fusiform gyrus.[31,36,37,38] Decreased brain volumes in structures outside the medial temporal lobe including posterior cingulate, lateral temporal lobe, medial parietal lobe (retrosplenial cortex, posterior cingulate, and precuneus) and parietal association cortex have also been described.[31,39]
Almeida et al. showed that ischemic heart disease (IHD) was associated with decreased gray matter in left cingulate, right inferior frontal gyrus, right middle temporal and frontal gyrus, inferior and precentral frontal gyrus and occipital and parietal regions involving left precuneus.[40] In another study, CHD participants showed decreased grey matter volume in the left medial frontal lobe (including the cingulate), precentral and postcentral cortex, right temporal lobe and left middle temporal gyrus, and left precuneus and posterior cingulate.[41]
The above mentioned studies attempted to correlate volumetric changes in brains of the MCI patients with CAD only based on history of IHD. To our best of knowledge, there has not been any study that evaluated the correlation of an objective CAD index, as an independent variable, to changes of brain volumes in MCI.
In patients with CAD, the vascular health state may be directly assessed by coronary angiography as an objective, reliable, and valid method. Several scoring systems for coronary angiography have been determined to estimate the extent of coronary artery involvement. One scoring system is “Gensini scoring method” that evaluates the severity and location of stenosis; and efficiency of collateral arteries.[42]
In this study, we compared brain volumes of patients with MCI and control subjects in individuals with recent coronary artery angiography. We investigated the relationship between coronary angiography findings and volumes of specific brain regions.
MATERIALS AND METHODS
All individuals (N = 1625) who were admitted to cardiac catheterization facilities of Sina and Nour Hospitals of Isfahan, Iran, from March 21st to October 20th 2012. The patients were screened through telephone calls for inclusion and exclusion criteria. The inclusion criteria included, age (60 years or more), education (primary or higher levels), and history of coronary angiography during the past 12 months. Patients with a history of head trauma, serious medical or neurological disease, major psychiatric disorders, substance-related disorders and dementia were not included. Any Information regarding the patient's history of diabetes, hypertension, smoking, hyperlipidemia, or a family history of Alzheimer's disease were also recorded. The General Health Questionnaires (GHQ) was completed for all subjects.[43] In addition, blood pressure, pulse rate, height, weight and body mass index were recorded, and biochemical tests including fasting blood glucose, creatinine, serum triglycerides, and total cholesterol were performed.
A total of 143 subjects, who met the above criteria has undergone a neuropsychiatry interview, Mini-Mental State Examination[44] and assessment with the Neuropsychiatry Unit COGnitive assessment tool (NUCOG).[45] Patients who were diagnosed with MCI, based on neuropsychiatry interview would have NUCOG scores between 75 and 86.5 to confirm the diagnosis of MCI. Cognitively normal subjects, based on the clinical interview, had NUCOG scores of greater than 86.5.[46] Finally, 20 patients were selected and one for one with 20 cognitively normal subjects [Figure 1].
Figure 1.
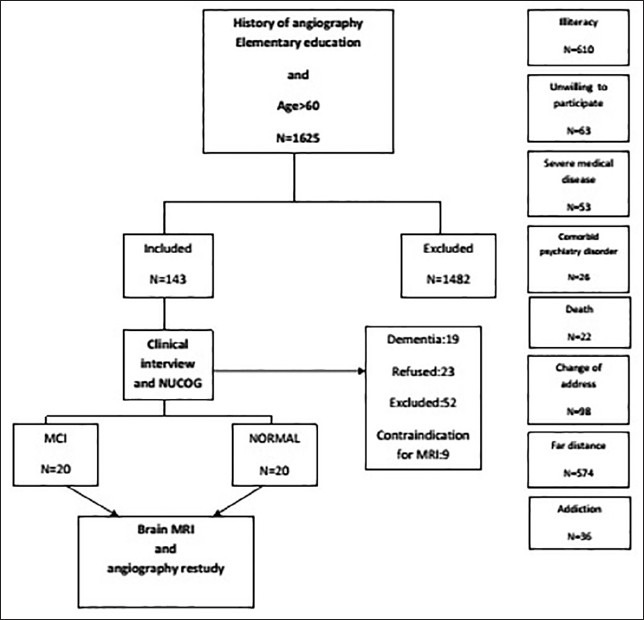
Flowchart of the study
Coronary angiography
Video records of coronary angiography were re-investigated by an expert cardiologist. The Gensini score was calculated through multiplying the severity of stenosis by the segment location by collateral adjustment factor. A higher Gensini score is associated with more extensive CAD.[42]
Magnetic resonance imaging scanning
Magnetic resonance imaging was performed using a 1.5 Tesla MRI Siemens Avanto scanner system (Siemens, Germany). The following protocol was administered for obtaining T1-weighted magnetization-prepared, rapid gradient echo (MP-RAGE) scans, with thickness of 1.2 mm: Repetition time = 25 ms, echo time = 3.61 ms, flip angle = 8°, field of view = 240 × 240 mm2, matrix size = 192 × 192, voxel dimensions = 1.3 × 1.3 × 1.2 mm3, number of excitations = 1, and number of slices = 160.
Regions of Interests (ROI) for volumetric measurement were based on previous findings and comprised of the hippocampus, amygdala, ERC, para hippocampal gyrus, pars triangularis, cuneus, precuneus, fusiform gyrus, supra marginal gyrus, total cortex, temporal and parietal cortex and posterior cingulate gyrus.
Magnetic resonance imaging analysis
All MRI scans were processed using the FreeSurfer software package, available at http://www.surfer.nmr.mgh.harvard.edu. Multiple MP-RAGE MRI acquisitions for each participant were motion corrected, averaged and normalized to create a single image volume with relatively high contrast to noise. This averaged volume was used to locate the grey/white matter boundary (white matter surface) and this, in turn, was then used to locate the grey/CSF boundary (grey matter surface).[36]
The details of study involvement were discussed with all participants and written informed consent was obtained.
Statistical analysis
The analysis was performed using SPSS software version 16.0 (SPSS Inc., Chicago, Illinois, USA). Independent t-tests and Chi-square tests were used to compare baseline variables. Differences between patients and controls were tested using multiple analysis of co-variance for each ROI with controlling the confounding effects of intracranial volume.
Pearson correlation was used to identify the strength and direction of any correlation between Gensini scores and brain volumes; with statistical significance set at P < 0.05. The analysis was performed using SPSS software version 16.0. (Chicago, SPSS Inc).
RESULTS
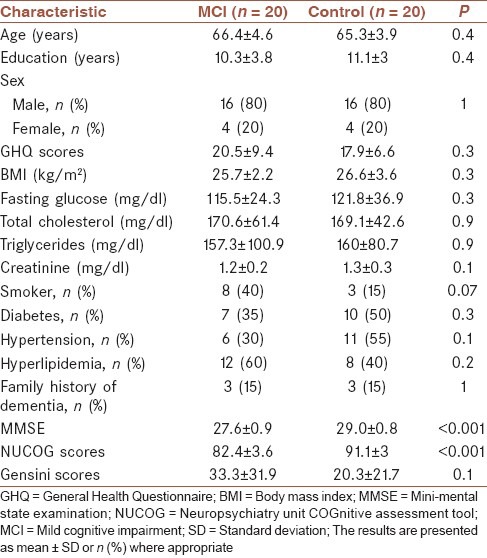
Twenty patients with MCI (16 men and 4 women) and 20 matched subjects with normal cognitive state (16 men and 4 women) were selected for this study. Baseline characteristics of which are summarized in Table 1. The two groups did not differ on age, gender, years of education, smoking, history of diabetes mellitus, hypertension, hyperlipidemia, family history of Alzheimer disease, body mass index, GHQ, serum total cholesterol, fasting blood glucose, triglyceride, and creatinine.
Table 1.
Patient demographics and cardiovascular risk factors
Comparisons of ROIs volumes between the two groups revealed significant differences in left fusiform (P = 0.039), left pars triangularis (P = 0.003) and left superior temporal gyrus (P = 0.009) after controlling for intracranial volumes. There were no significant differences between the two groups in volume of the hippocampus, amygdala, cuneus, precuneus, ERC, para hippocampal gyrus, supra marginal gyrus, total cortex, parietal cortex, temporal cortex, and posterior cingulate gyrus (P > 0.05).
Correlation coefficients between Gensini scores and volumes of ROIs in the MCI group revealed that increasing Gensini scores were associated with decreasing volumes of total cortical volume (P = 0.047, R = −0.4), left precuneus (P = 0.022, R = −0.5), right inferior parietal lobule (P = 0.011, R = −0.5) and left supra marginal gyrus (P = 0.035, R = −0.04). These associations were not found in the control group [Table 2].
Table 2.
Correlation between Gensini scores and region of interests volumes in MCI and control groups
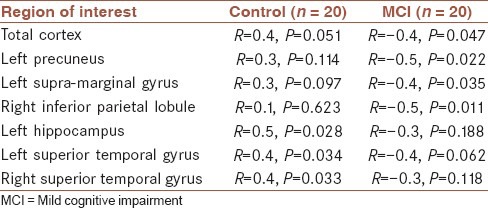
In the control group increasing Gensini scores was positively correlated with volumes of left hippocampus (P = 0.028, R = 0.5), left superior temporal gyrus (P = 0.034, R = 0.4) and right inferior temporal gyrus (P = 0.033, R = 0.4). These associations were not found in the MCI group [Table 2].
DISCUSSION
This is the first study to evaluate relationship between Gensini scores, as an objective scoring system of CAD and atrophy of individual anatomic ROI in MCI. Previous studies have revealed that the volume of left cingulate, right inferior frontal gyrus, inferior and precentral frontal gyrus, right and left middle temporal gyrus and parietal regions involving left precuneus, and post-central cortex may be reduced in patients with CAD. However, in these studies the diagnosis of CAD was based solely on history of IHD.[40,41]
One key finding of this study was the inverse correlation between Gensini scores and volumes of cortical volume (P = 0.047, R = −0.4), left precuneus (P = 0.022, R = −0.5), right inferior parietal lobule (P = 0.011, R = −0.5) and left supra marginal gyrus (P = 0.035, R = −0.04). The cognitively normal subjects did not demonstrate these correlations. Our imaging findings have shown that people with CAD display more volume reduction in the posterior association cortex and the precuneus. These regions have major bidirectional connections with the medial temporal lobes in primates, which plays an important role in the successful retrieval of memory traces.[47]
Surprisingly, we found that the Gensini scores revealed positive relations with left hippocampus (P = 0.028, R = 0.5), left superior temporal gyrus (P = 0.034, R = 0.4) and right inferior temporal gyrus (P = 0.033, R = 0.4) in the control group [Table 2]. One reason may be compensatory changes for subcortical vascular changes affecting white matter. Other possible explanation is that the controls may be in “pre-MCI” state. Thus, there might be early compensatory changes that involve expansion, before volume reductions appear as individuals with presumed latent cerebrovascular disease progress to MCI. Finally, as the apparent strength of associations between the Gensini score and regional brain volumes were in opposite directions, another suggestion may be a chance of collinearity. It means that both associations are linked to one or more other explanatory factors.
Anozodo showed that, in patients with CAD, gray matter volumes in the superior, medial and inferior frontal gyrus, superior and inferior parietal gyrus, middle and superior temporal gyrus and posterior cerebellum were decreased in comparison with healthy controls.[48] Jefferson reported that the cardiac Index (cardiac output/body surface area) was positively associated with total brain volume and inversely associated with lateral ventricular volume, suggesting that reduction in subcortical white matter volume may also be reduced in these patients. In patients with IHD, gray matter loss were similar, but to a lesser extensive pattern.[49] Zheng evaluated 74 cognitively normal individuals with neuropsychological assessments. Zheng revealed that CAD was associated with greater declines in global memory, verbal memory, and executive cognition. Surprisingly, this association was found again after controlling hippocampal and cortical gray matter volumes. Zheng concluded that grey matter volume changes in MRI could not fully explain the association between CAD and cognitive decline.[50]
Comparisons of the volumes revealed significant differences in between the 2 groups in left fusiform (P = 0.039), left pars triangularis (P = 0.003) and left superior temporal gyrus (P = 0.009) when intracranial volumes were controlled. Like our findings, Desikan reported significant differences between the MCI patients and cognitively normal subjects in volumes of the superior temporal gyrus. However, this study demonstrated additional decreased volumes in lateral occipital cortex, thickness of isthmus of cingulate cortex, supra marginal gyrus, ERC, inferior parietal lobule, inferior temporal gyrus, lingual cortex, middle temporal gyrus, parahippocampal gyrus, amygdala, hippocampus and temporal pole in MCI group.[36] In Han et al.'s study, significantly decreased gray matter volume was demonstrated, in the amnestic MCI group, predominantly in the bilateral prefrontal, left temporal and posterior cingulate cortex.[51] Decreased volume of the left fusiform gyrus was also reported by Trividi in MCI patients compared with controls. Unlike our study, Trividi reported that MCI patients had atrophy in right inferior and middle temporal gyrus; and anterior medial temporal lobe.[39]
In our study, hippocampal volumes did not differ between MCI and control groups. Several studies have reported bilateral hippocampal atrophy in MCI patients.[31,36,37] However, there have been a number of conflicting findings. Menezes et al.[52] and Hänggi et al.[53] did not find any difference in hippocampal volume in MCI as compared to control the group. Muller found significant lower volume only in the left hippocampus,[54] and Zhang et al. reported smaller hippocampal volume only in the right side.[55] There are studies that reported significant hippocampal atrophy only in a-MCI, but not na-MCI.[14,56] Siuda et al. demonstrated that hippocampal volume in the control group differed significantly from the a-MCI group, but not the na-MCI group.[14] Vos et al. reported that hippocampal atrophy might not be as sensitive for early diagnosis of AD in na-MCI compared with a-MCI.[56] Therefore, decreased hippocampal volume may be considered as a specific biomarker of a-MCI. As our MCI patients consisted of both types of a-MCI and na-MCI, the heterogeneity of our sample may have masked any correlation between hippocampaI atrophy and a-MCI patients in our sample.
This study, however, has a number of limitations. First, the sample size is small, which reduces confidence in the validity of the observed associations. The study population is also relatively young, and the relevance of the observations to older patients is uncertain. Second, no information is provided about left ventricular function or prevalence of heart failure in the study population. Third, it is a case-control study; prospective cohort study that follows patients who transition to MCI may reveal the predictive validity of a number of our variables. Fourth, we did not stratify MCI subtypes for better classification. Furthermore, all participants have been treated for IHD, hypertension, hyperlipidemia, and diabetes. These interventions could alter or attenuate the effect of cardiovascular risk factors on cognitive performance. Finally, we did not consider the duration of CAD, which may exert a progressive/cumulative effect on regional brain volume.
CONCLUSION
In summary, our study shows that patients with MCI, who has a greater extent of coronary stenosis experience more loss of gray matter in some specific brain regions that are relevant to cognitive function. This was not the case for cognitively normal subjects. Our results are consistent with the observation that evidence of active degenerative process in the brain of patients with CAD. Future longitudinal studies should aim to elucidate progressive nature and the biological link CAD to decreased cerebral GM and cognitive impairment in MCI.
AUTHOR'S CONTRIBUTIONS
MB: Contributed in the conception of the work, designing the proposal, conducting the work, analysis of the data, interpretation of the results, drafting of the manuscript, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. HA: Contributed in the conception of the work, conducting the work, analysis of the data, drafting and revision of the article, approval of the final version of the manuscript, and agreed for all aspects of the work. FZ: Contributed in the conception of the work, conducting the work, drafting and revision of the article, approval of the final version of the manuscript, and agreed for all aspects of the work. MW: Contributed in the conception of the work, analysis of the data, interpretation of the results, drafting of the manuscript, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. DV: Contributed in the conception of the work, interpretation of the results, drafting of the manuscript, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MH: Contributed in the conception of the work, designing the proposal, interpretation of the results, drafting of the manuscript, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MRM: Contributed in the conception of the work, designing the proposal, analysis of the data, interpretation of the results, drafting of the manuscript, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
ACKNOWLEDGMENTS
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
This study was scientifically and ethically discussed and approved by the Deputy of Research and Technology, Isfahan University of Medical Sciences, Isfahan, Iran.
This project was the thesis of FZ, which was funded by the Isfahan University of Medical Sciences (Research number 391052). The sponsor did not have any role in the design, methods, subject recruitment, data collections, analysis and preparation of the paper. This study was scientifically and ethically discussed and approved by the Deputy of Research and Technology, Isfahan University of Medical Sciences, Isfahan, Iran.
The investigators thank the staff of Shafa Imaging Center, Isfahan, Iran, for their generous contribution.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Geneva: World Health Organization; 2003. World Health Organization. World Health Report. 2003. Shaping the Future. Open URL. [Google Scholar]
- 2.World Health Organization. Global Health and Aging. 2011. [Last accessed on 2013 Nov 12]. Available from: http://www.who.int/aging/publications/globalhealth.pdf .
- 3.Apostolova LG, Green AE, Babakchanian S, Hwang KS, Chou YY, Toga AW, et al. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer Disease. Alzheimer Dis Assoc Disord. 2012;26:17–27. doi: 10.1097/WAD.0b013e3182163b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Kovacevic S, Rafii MS, Brewer JB Alzheimer's Disease Neuroimaging Initiative. High-throughput, fully automated volumetry for prediction of MMSE and CDR decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2009;23:139–45. doi: 10.1097/WAD.0b013e318192e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 7.He J, Farias S, Martinez O, Reed B, Mungas D, Decarli C. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch Neurol. 2009;66:1393–9. doi: 10.1001/archneurol.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEvoy LK, Edland SD, Holland D, Hagler DJ, Jr, Roddey JC, Fennema-Notestine C, et al. Neuroimaging enrichment strategy for secondary prevention trials in Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24:269–77. doi: 10.1097/WAD.0b013e3181d1b814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitehair DC, Sherzai A, Emond J, Raman R, Aisen PS, Petersen RC, et al. Influence of apolipoprotein E varepsilon4 on rates of cognitive and functional decline in mild cognitive impairment. Alzheimers Dement. 2010;6:412–9. doi: 10.1016/j.jalz.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luck T, Luppa M, Briel S, Riedel-Heller SG. Incidence of mild cognitive impairment: A systematic review. Dement Geriatr Cogn Disord. 2010;29:164–75. doi: 10.1159/000272424. [DOI] [PubMed] [Google Scholar]
- 11.Risacher SL, Kim S, Shen L, Nho K, Foroud T, Green RC, et al. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI) Front Aging Neurosci. 2013;5:11. doi: 10.3389/fnagi.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. Cardiac disease associated with increased risk of nonamnestic cognitive impairment: Stronger effect on women. JAMA Neurol. 2013;70:374–82. doi: 10.1001/jamaneurol.2013.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monastero R, Palmer K, Qiu C, Winblad B, Fratiglioni L. Heterogeneity in risk factors for cognitive impairment, no dementia: Population-based longitudinal study from the Kungsholmen Project. Am J Geriatr Psychiatry. 2007;15:60–9. doi: 10.1097/01.JGP.0000229667.98607.34. [DOI] [PubMed] [Google Scholar]
- 14.Siuda J, Gorzkowska A, Opala G, Ochudlo S. Vascular risk factors and intensity of cognitive dysfunction in MCI. J Neurol Sci. 2007;257:202–5. doi: 10.1016/j.jns.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Roger VL, Jacobsen SJ, Weston SA, Goraya TY, Killian J, Reeder GS, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–8. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 16.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer's disease: Inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–46. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 17.Ott A, Andersen K, Dewey ME, Letenneur L, Brayne C, Copeland JR, et al. Effect of smoking on global cognitive function in nondemented elderly. Neurology. 2004;62:920–4. doi: 10.1212/01.wnl.0000115110.35610.80. [DOI] [PubMed] [Google Scholar]
- 18.Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer's disease in later life: Longitudinal, population based study. BMJ. 2001;322:1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Torre JC. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke. 2002;33:1152–62. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 20.Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–4. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 21.Langa KM, Foster NL, Larson EB. Mixed dementia: Emerging concepts and therapeutic implications. JAMA. 2004;292:2901–8. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- 22.Evered LA, Silbert BS, Scott DA, Maruff P, Laughton KM, Volitakis I, et al. Plasma amyloid beta42 and amyloid beta40 levels are associated with early cognitive dysfunction after cardiac surgery. Ann Thorac Surg. 2009;88:1426–32. doi: 10.1016/j.athoracsur.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Devapalasundarum AN, Silbert BS, Evered LA, Scott DA, MacIsaac AI, Maruff PT. Cognitive function in patients undergoing coronary angiography. Heart Asia. 2010;2:75–9. doi: 10.1136/ha.2009.001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosengart TK, Sweet J, Finnin EB, Wolfe P, Cashy J, Hahn E, et al. Neurocognitive functioning in patients undergoing coronary artery bypass graft surgery or percutaneous coronary intervention: Evidence of impairment before intervention compared with normal controls. Ann Thorac Surg. 2005;80:1327–34. doi: 10.1016/j.athoracsur.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 25.Zulli R, Nicosia F, Borroni B, Agosti C, Prometti P, Donati P, et al. Increased prevalence of silent myocardial ischaemia and severe ventricular arrhythmias in untreated patients with Alzheimer's disease and mild cognitive impairment without overt coronary artery disease. Clin Neurol Neurosurg. 2008;110:791–6. doi: 10.1016/j.clineuro.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging. 2010;31:1894–902. doi: 10.1016/j.neurobiolaging.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins IJ, Hone E, Foster JK, Sünram-Lea SI, Gnjec A, Fuller SJ, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–36. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 28.Freiheit EA, Hogan DB, Eliasziw M, Patten SB, Demchuk AM, Faris P, et al. A dynamic view of depressive symptoms and neurocognitive change among patients with coronary artery disease. Arch Gen Psychiatry. 2012;69:244–55. doi: 10.1001/archgenpsychiatry.2011.1361. [DOI] [PubMed] [Google Scholar]
- 29.Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging. 2012;33:1979–87. doi: 10.1016/j.neurobiolaging.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen L, Kim Q, Qi Y, Inlow M, Swaminathan S, Nho K, et al. Identifying Neuroimaging and Proteomic Biomarkers for MCI and AD via the Elastic Net. Multimodal Brain Image Analysis. Lect Notes Comput Sci. 2011;7012:27–34. doi: 10.1007/978-3-642-24446-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ries ML, Carlsson CM, Rowley HA, Sager MA, Gleason CE, Asthana S, et al. Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: A review. J Am Geriatr Soc. 2008;56:920–34. doi: 10.1111/j.1532-5415.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker JT, Davis SW, Hayashi KM, Meltzer CC, Toga AW, Lopez OL, et al. Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Arch Neurol. 2006;63:97–101. doi: 10.1001/archneur.63.1.97. [DOI] [PubMed] [Google Scholar]
- 33.Hämäläinen A, Tervo S, Grau-Olivares M, Niskanen E, Pennanen C, Huuskonen J, et al. Voxel-based morphometry to detect brain atrophy in progressive mild cognitive impairment. Neuroimage. 2007;37:1122–31. doi: 10.1016/j.neuroimage.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hänninen T, Laakso MP, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25:303–10. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 35.Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;71:441–7. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, et al. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain. 2009;132:2048–57. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;19:1055–64. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 38.Macdonald KE, Bartlett JW, Leung KK, Ourselin S, Barnes J ADNI investigators. The value of hippocampal and temporal horn volumes and rates of change in predicting future conversion to AD. Alzheimer Dis Assoc Disord. 2013;27:168–73. doi: 10.1097/WAD.0b013e318260a79a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trivedi MA, Wichmann AK, Torgerson BM, Ward MA, Schmitz TW, Ries ML, et al. Structural MRI discriminates individuals with Mild cognitive impairment from age-matched controls: A combined neuropsychological and voxel based morphometry study. Alzheimers Dement. 2006;2:296–302. doi: 10.1016/j.jalz.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida OP, Garrido GJ, Beer C, Lautenschlager NT, Arnolda L, Flicker L. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J. 2012;33:1769–76. doi: 10.1093/eurheartj/ehr467. [DOI] [PubMed] [Google Scholar]
- 41.Almeida OP, Garrido GJ, Beer C, Lautenschlager NT, Arnolda L, Lenzo NP, et al. Coronary heart disease is associated with regional grey matter volume loss: Implications for cognitive function and behaviour. Intern Med J. 2008;38:599–606. doi: 10.1111/j.1445-5994.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 42.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 43.Malakouti SK, Fatollahi P, Mirabzadeh A, Zandi T. Reliability, validity and factor structure of the GHQ-28 used among elderly Iranians. Int Psychogeriatr. 2007;19:623–34. doi: 10.1017/S1041610206004522. [DOI] [PubMed] [Google Scholar]
- 44.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 45.Walterfang M, Siu R, Velakoulis D. The NUCOG: Validity and reliability of a brief cognitive screening tool in neuropsychiatric patients. Aust N Z J Psychiatry. 2006;40:995–1002. doi: 10.1080/j.1440-1614.2006.01923.x. [DOI] [PubMed] [Google Scholar]
- 46.Barekatain M, Walterfang M, Behdad M, Tavakkoli M, Mahvari J, Maracy MR, et al. Validity and reliability of the Persian language version of the neuropsychiatry unit cognitive assessment tool. Dement Geriatr Cogn Disord. 2010;29:516–22. doi: 10.1159/000313981. [DOI] [PubMed] [Google Scholar]
- 47.Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–49. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Anazodo UC, Shoemaker JK, Suskin N, St Lawrence KS. An investigation of changes in regional gray matter volume in cardiovascular disease patients, pre and post cardiovascular rehabilitation. Neuroimage Clin. 2013;3:388–95. doi: 10.1016/j.nicl.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, et al. Cardiac index is associated with brain aging: The Framingham Heart Study. Circulation. 2010;122:690–7. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng L, Mack WJ, Chui HC, Heflin L, Mungas D, Reed B, et al. Coronary artery disease is associated with cognitive decline independent of changes on magnetic resonance imaging in cognitively normal elderly adults. J Am Geriatr Soc. 2012;60:499–504. doi: 10.1111/j.1532-5415.2011.03839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han Y, Lui S, Kuang W, Lang Q, Zou L, Jia J. Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS One. 2012;7:e28664. doi: 10.1371/journal.pone.0028664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menezes TL, Andrade-Valença LP, Valença MM. Magnetic resonance imaging study cannot individually distinguish individuals with mild cognitive impairment, mild Alzheimer's disease, and normal aging. Arq Neuropsiquiatr. 2013;71:207–12. doi: 10.1590/0004-282x20130003. [DOI] [PubMed] [Google Scholar]
- 53.Hänggi J, Streffer J, Jäncke L, Hock C. Volumes of lateral temporal and parietal structures distinguish between healthy aging, mild cognitive impairment, and Alzheimer's disease. J Alzheimers Dis. 2011;26:719–34. doi: 10.3233/JAD-2011-101260. [DOI] [PubMed] [Google Scholar]
- 54.Müller MJ, Greverus D, Dellani PR, Weibrich C, Wille PR, Scheurich A, et al. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage. 2005;28:1033–42. doi: 10.1016/j.neuroimage.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–9. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vos SJ, van Rossum IA, Verhey F, Knol DL, Soininen H, Wahlund LO, et al. Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology. 2013;80:1124–32. doi: 10.1212/WNL.0b013e318288690c. [DOI] [PubMed] [Google Scholar]


