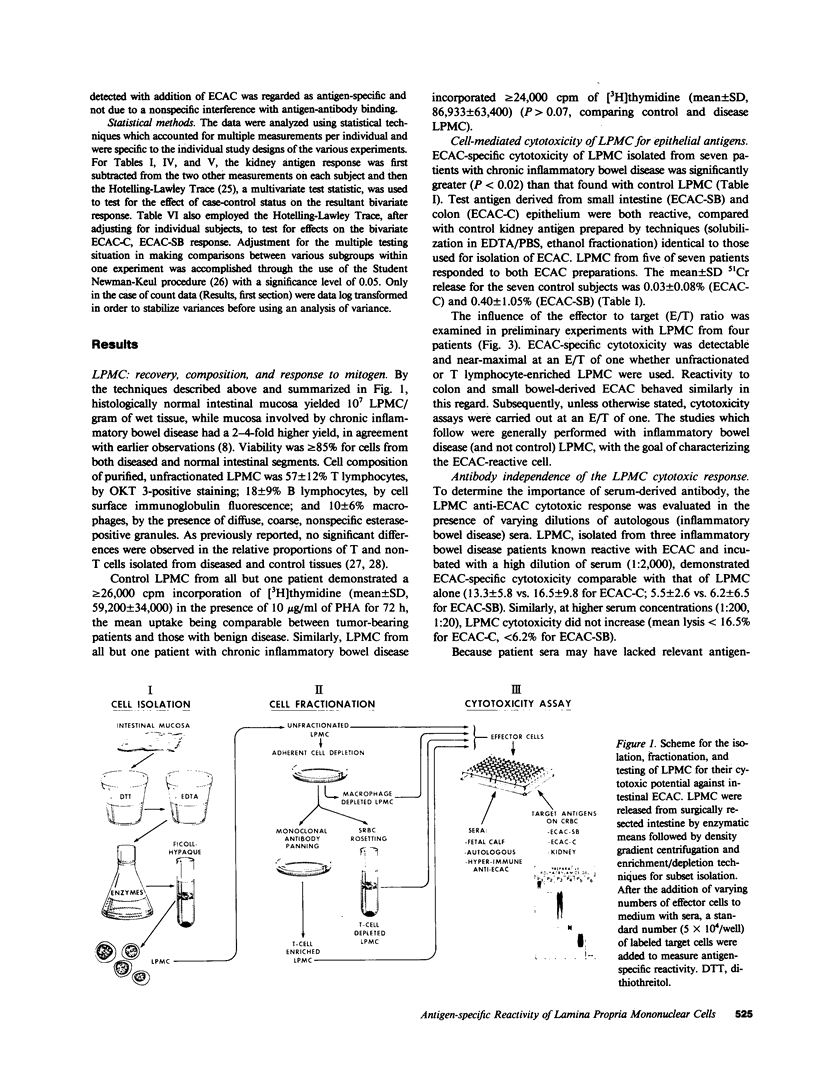
Abstract
To explore the auto-reactive potential of cells infiltrating the gut mucosa in idiopathic chronic inflammatory bowel disease, intestinal lamina propria mononuclear cells (LPMC) were isolated, characterized morphologically and phenotypically, and evaluated for antigen-specific reactivity. The last was assessed by quantitating LPMC cytotoxic capabilities against purified, aqueous-soluble, organ-specific epithelial cell-associated components (ECAC) characterized previously. Enzyme-isolated inflammatory bowel disease LPMC were constituted primarily by T lymphocytes (57 +/- 12% OKT 3-positive), B lymphocytes (18 +/- 9% surface immunoglobulin-positive), and macrophages (11 +/- 6% esterase-positive), and were responsive to phytohemagglutinin (mean uptake 86,933 cpm/5 X 10(5) cells). LPMC present in abnormal segments from 71% of patients with chronic inflammatory bowel disease were cytotoxic for ECAC isolated from colon (12.5 +/- 8.9% specific lysis) and small bowel (7.1 +/- 6.5%), but not for kidney control antigen (0.8 +/- 1.1%) isolated in a manner analogous to that used for ECAC (P less than 0.02). In contrast, despite comparable responses to phytohemagglutinin (mean uptake 59,200 cpm/5 X 10(5) cells), LPMC from histologically normal mucosa of patients with benign (adult megacolon, Hirshsprung's disease, diverticulosis) or malignant disease failed to lyse indicator cells labeled with colon-derived ECAC (0.3 +/- 0.08%), small bowel-derived ECAC (0.4 +/- 1.11%), or kidney antigen (0.29 +/- 0.79%). LPMC reactivity for individual gel-purified macromolecules of small bowel-derived ECAC (designated as the "P" series of components) was greatest against component P1 (by 2-3-fold), but was detectable against three other purified components as well. The addition of patient's serum did not enhance cytotoxicity to ECAC. Characterization of the cytotoxic cell showed that it was nonadherent to plastic surfaces, bore T lymphocyte-specific markers detectable by OKT 11 and OKT 3 monoclonal antibodies, and could be depleted by removal of cells with receptors for sheep erythrocytes. ECAC-specific reactivity was markedly reduced (greater than 93%) in most experiments when LPMC were preincubated for 1 h with ECAC. These data support the concept that autosensitization to several epithelial cell-associated components has occurred in patients with chronic inflammatory bowel disease, and provide initial evidence that antigen-specific, cell-mediated mechanisms may play a role in the pathogenesis of these disorders.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson R. A., Cook S. L., Roche J. K. Sensitization to epithelial antigens in chronic mucosal inflammatory disease. I. Purification, characterization, and immune reactivity of murine epithelial cell-associated components (ECAC). J Immunol. 1983 Dec;131(6):2796–2804. [PubMed] [Google Scholar]
- Bartnik W., ReMine S. G., Chiba M., Thayer W. R., Shorter R. G. Isolation and characterization of colonic intraepithelial and lamina proprial lymphocytes. Gastroenterology. 1980 May;78(5 Pt 1):976–985. [PubMed] [Google Scholar]
- Bockman D. E., Cooper M. D. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am J Anat. 1973 Apr;136(4):455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Organ specificity of lymphocyte migration: mediation by highly selective lymphocyte interaction with organ-specific determinants on high endothelial venules. Eur J Immunol. 1980 Jul;10(7):556–561. doi: 10.1002/eji.1830100713. [DOI] [PubMed] [Google Scholar]
- Chiba M., Bartnik W., ReMine S. G., Thayer W. R., Shorter R. G. Human colonic intraepithelial and lamina proprial lymphocytes: cytotoxicity in vitro and the potential effects of the isolation method on their functional properties. Gut. 1981 Mar;22(3):177–186. doi: 10.1136/gut.22.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell P., Dawson J. R. Dimeric and monomeric forms of HL-A antigens solubilized by detergent. J Immunol. 1975 Jan;114(1 Pt 2):523–525. [PubMed] [Google Scholar]
- Das K. M., Dubin R., Nagai T. Isolation and characterization of colonic tissue-bound antibodies from patients with idiopathic ulcerative colitis. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4528–4532. doi: 10.1073/pnas.75.9.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dombal F. T., Burton I. L., Clamp S. E., Goligher J. C. Short-term course and prognosis of Crohn's disease. Gut. 1974 Jun;15(6):435–443. doi: 10.1136/gut.15.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchuk Z. M., Barnhard E., Machado I. Human colonic mononuclear cells: studies of cytotoxic function. Gut. 1981 Apr;22(4):290–294. doi: 10.1136/gut.22.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C., Battisto J. R., Farmer R. G. Gut mucosal lymphocytes in inflammatory bowel disease: isolation and preliminary functional characterization. Dig Dis Sci. 1979 Sep;24(9):705–717. doi: 10.1007/BF01314469. [DOI] [PubMed] [Google Scholar]
- Fiocchi C., Battisto J. R., Farmer R. G. Studies on isolated gut mucosal lymphocytes in inflammatory bowel disease. Detection of activated T cells and enhanced proliferation to Staphylococcus aureus and lipopolysaccharides. Dig Dis Sci. 1981 Aug;26(8):728–736. doi: 10.1007/BF01316863. [DOI] [PubMed] [Google Scholar]
- Fiocchi C., Youngman K. R., Farmer R. G. Immunoregulatory function of human intestinal mucosa lymphoid cells: evidence for enhanced suppressor cell activity in inflammatory bowel disease. Gut. 1983 Aug;24(8):692–701. doi: 10.1136/gut.24.8.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Grol M., Schumacher K. Purification and biochemical characterization of human liver-derived inhibitory protein (LIP). J Immunol. 1983 Jan;130(1):323–326. [PubMed] [Google Scholar]
- Hagopian A., Jackson J. J., Carlo D. J., Limjuco G. A., Eylar E. H. Experimental allergic aspermatogenic orchitis. III. Isolation of spermatozoal glycoproteins and their role in allergic aspermatogenic orchitis. J Immunol. 1975 Dec;115(6):1731–1743. [PubMed] [Google Scholar]
- KIRSNER J. B., ELCHLEPP J. G., GOLDGRABER M. B., ABLAZA J., FORD H. Production of an experimental ulcerative "colitis" in rabbits. Arch Pathol. 1959 Oct;68:392–408. [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Lagercrantz R., Hammarström S., Perlmann P., Gustafsson B. E. Immunological studies in ulcerative colitis. IV. Origin of autoantibodies. J Exp Med. 1968 Dec 1;128(6):1339–1352. doi: 10.1084/jem.128.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Yam L. T., Lam K. W. Acid phosphatase isoenzyme in human leukocytes in normal and pathologic conditions. J Histochem Cytochem. 1970 Jul;18(7):473–481. doi: 10.1177/18.7.473. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Franklin G. O., Jenkins K. M., Kodner I. J., Nash G. S., Weinrieb I. J. Human intestinal mononuclear cells. I. Investigation of antibody-dependent, lectin-induced, and spontaneous cell-mediated cytotoxic capabilities. Gastroenterology. 1980 Jan;78(1):47–56. [PubMed] [Google Scholar]
- Männel D., Mayer H. Isolation and chemical characterization of the enterobacterial common antigen. Eur J Biochem. 1978 May 16;86(2):361–370. doi: 10.1111/j.1432-1033.1978.tb12318.x. [DOI] [PubMed] [Google Scholar]
- Nagai T., Das K. M. Detection of colonic antigen(s) in tissues from ulcerative colitis using purified colitis colon tissue-bound IgG (CCA-IgG). Gastroenterology. 1981 Sep;81(3):463–470. [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- RICHARDSON G. S., LESKOWITZ S. An attempt at production of autoimmunity to tissue of the gastrointestinal tract. Proc Soc Exp Biol Med. 1961 Feb;106:357–359. doi: 10.3181/00379727-106-26337. [DOI] [PubMed] [Google Scholar]
- Rabin B. S. Duodenum-, ileum- and colon-specific antigens. Int Arch Allergy Appl Immunol. 1976;50(2):133–141. doi: 10.1159/000231490. [DOI] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- Roche J. K., Cook S. L., Day E. D. Goblet cell glycoprotein: an organ-specific antigen for gut. Isolation, tissue localization and immune response. Immunology. 1981 Dec;44(4):799–810. [PMC free article] [PubMed] [Google Scholar]
- Roche J. K., Cook S. R., Day E. D. Cellular cytotoxicity and gastrointestinal inflammation in inbred rats: induction with gut organ-specific antigens. Immunology. 1981 Nov;44(3):489–497. [PMC free article] [PubMed] [Google Scholar]
- Roche J. K., Day E. D., Hill H. D. Rabbit antibodies to ovine-submaxillary mucin. Detection, specificity and cross-reactivity. Immunochemistry. 1978 May;15(5):339–343. doi: 10.1016/0161-5890(78)90096-2. [DOI] [PubMed] [Google Scholar]
- Roche J. K., Varitek V. A., Hill H. D., Day E. D. Specificity and T-lymphocyte dependence of the humoral immune response in the rat to purified ovine and porcine mucins. Mol Immunol. 1979 Aug;16(8):609–619. doi: 10.1016/0161-5890(79)90124-x. [DOI] [PubMed] [Google Scholar]
- Schachter H., Kirsner J. B. Definitions of inflammatory bowel disease of unknown etiology. Gastroenterology. 1975 Mar;68(3):591–600. [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Shorter R. G., McGill D. B., Bahn R. C. Cytotoxicity of mononuclear cells for autologous colonic epithelial cells in colonic diseases. Gastroenterology. 1984 Jan;86(1):13–22. [PubMed] [Google Scholar]
- Shorter R. G., McGill D. B., Bahn R. C. Cytotoxicity of mononuclear cells for autologous colonic epithelial cells in colonic diseases. Gastroenterology. 1984 Jan;86(1):13–22. [PubMed] [Google Scholar]
- Stobo J. D., Tomasi T. B., Huizenga K. A., Spencer R. J., Shorter R. G. In vitro studies of inflammatory bowel disease. Surface receptors of the mononuclear cell required to lyse allogeneic colonic epithelial cells. Gastroenterology. 1976 Feb;70(2):171–176. [PubMed] [Google Scholar]
- TRUELOVE S. C., WITTS L. J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955 Oct 29;2(4947):1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher C., Wild G. C., Tung K. S. Acrosomal autoantigens of guinea pig sperm. I. The purification of an aspermatogenic protein, AP2. J Immunol. 1983 Jan;130(1):317–322. [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]
- Watson D. W., Quigley A., Bolt R. J. Effect of lymphocytes from patients with ulcerative colitis on human adult colon epithelial cells. Gastroenterology. 1966 Dec;51(6):985–993. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]