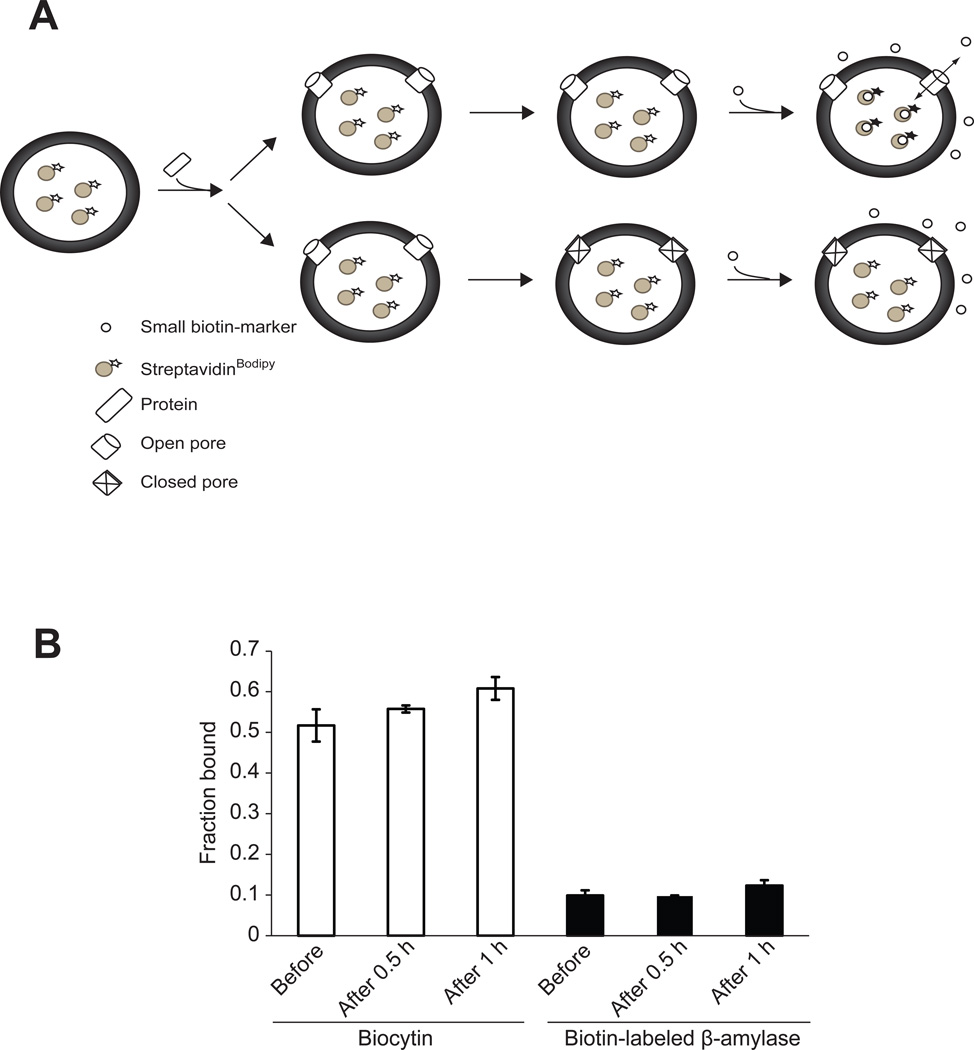
Figure 2. VP4 forms stable and size selective membrane pores.
(A) Scheme showing the pore stability assay employed. Pore stability was analyzed by employing a fluorescence-based spectroscopic assay, which detected the binding of streptavidinBodipy with fluorescence enhancers after the addition of the enhancer after incubation with VP4. The passage of biocytin (~1 nm diameter), biotin-labeled β-amylase (~5 nm diameter), or streptavidinBodipy (~5 nm diameter) through the pores formed by GST-VP4 was measured as detailed in Experimental Procedures. When the liposomes encapsulating streptavidinBodipy are used and the biotin-labeled molecules are externally added following incubation with VP4, fluorescence enhancement will be detected only if VP4 formed stable pores thereby allowing interaction of streptavidinBodipy and the biotin markers (top scenario). On the other hand, if VP4 does not form stable pores and closes after a brief period of time then the fluorescence will not increase when the enhancer is added after incubation with GST-VP4 (bottom scenario). The size of the pore will dictate which molecules can cross the membrane. (B) The stability of the pores was examined by measuring the increase in the fluorescence intensity of encapsulated streptavidinBodipy when the enhancers biocytin or biotin-labeled β-amylase were present in the external buffer solution either before the addition of VP4, added after incubation for 0.5 hr or 1 hr with VP4. Only biocytin was able to diffuse through the formed pores. The pores formed by VP4 remained open after incubation for 1 hr. The total lipid concentration was 100 µM, and the concentration of the protein was 232 nM. Each data point shows the average of at least two independent measurements with error bars representing standard deviations.