Abstract
Current polymer terminology only describes very simple copolymer structures such as block, graft, alternating periodic, or statistical copolymers. This restricted vocabulary implies that copolymers exhibit either segregated (i.e., block and graft), regular (i.e., alternating and periodic), or uncontrolled (i.e., statistical or random) comonomer sequence distributions. This standard classification does not include many new types of sequence-controlled copolymers that have been reported in recent years. In this context, the present viewpoint describes a new category of copolymers: aperiodic copolymers. Such structures can be defined as copolymers in which monomer sequence distribution is not regular but follows the same arrangement in all chains. The term aperiodic can be used to describe encoded comonomer sequences in monodisperse sequence-defined copolymers but also the block sequence of some multiblock copolymers. These new types of copolymers open up very interesting perspectives for the design of complex materials. Some recent relevant literature on the topic is discussed herein.
In 1945, Erwin Schrödinger introduced in his famous book “What is Life” the idea of an aperiodic crystal to describe genetic material.1 This was a remarkably visionary description, given the knowledge that was available at that time about the structure of macromolecules. Inspired by the book of Schrödinger, Watson and Crick confirmed a few years later that genetic information is encoded in the aperiodic sequences of DNA chains.2,3 Since then, it is universally admitted that the diversity and complexity of biology is linked to the comonomer sequences of biopolymers. There would be no genetic code, no evolution, and no living matter if DNA chains would be simple periodic structures.
In sharp contrast to the field of molecular biology, the notion of aperiodicity is almost fully ignored in synthetic polymer chemistry. Manmade copolymers that have been discovered and studied during the last 80 years are, in general, very simple structures. This situation is directly reflected by the terminology that can be found in standard polymer textbooks. For instance, the IUPAC official nomenclature for copolymers includes the following subclasses: block, graft, alternating periodic, random, and statistical (Figure 1).4,5 Block and graft copolymers are macromolecules composed of segments of different chemical nature (i.e., segmented copolymers). The terms alternating and periodic describe copolymers with regular sequences of comonomers such as (AB)n, (AAB)n, or (ABC)n. All other situations fall somehow in the broad category of statistical/random copolymers. This is a very incomplete description of what polymer chemistry can currently achieve. Some terms and categories are clearly missing here. Indeed, with the recent development of sequence-controlled polymerization methods, a wide variety of new copolymer structures, which do not belong in the aforementioned categories, have been synthesized and reported.6−8 Of course, some sequence-controlled polymers exhibit regular primary structures. As discussed in a recent review,6 alternating and periodic copolymers belong to the broad category of sequence-controlled macromolecules. However, many other sequence-controlled polymers do not exhibit periodic sequences, and such structures are probably the most interesting ones.10 Such aperiodic macromolecules are copolymers, in which comonomer sequences are intentionally distributed in a nonperiodic arrangement. However, the word “intentionally” is probably not fully appropriate to describe these structures because some natural aperiodic copolymers (e.g., DNA) were not created intentionally. A better definition is probably the following:
Figure 1.
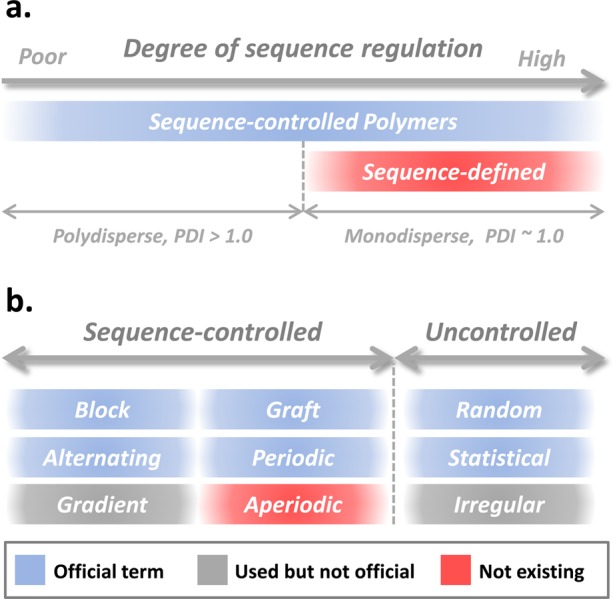
(a) Proposed definition for sequence-controlled and sequence-defined copolymers.9 (b) Some official (blue), nonofficial (gray), and nonexisting (red) terms to denote synthetic copolymers. Note that the color code is only valid for (b). The colors displayed in (a) are used for aesthetic reasons only.
Aperiodic copolymers are copolymers in which monomer sequence distribution is not regular but follows the same arrangement in all chains.
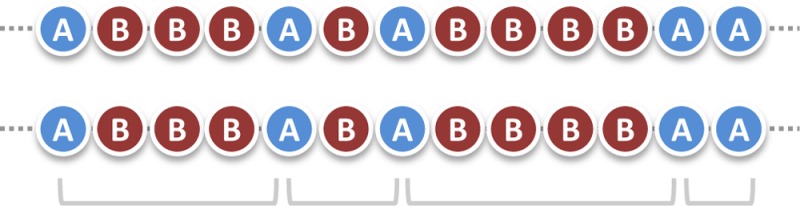
In this definition, the words “in all chains” are probably the most important ones. This is what makes the difference between an aperiodic copolymer and a random copolymer.11 In an aperiodic copolymer, the same monomer arrangement should be found in all chains, whereas in random copolymers, chain-to-chain deviations may exist. Aperiodicity is not randomness. Aperiodicity is not statistical either, because the word statistical is restrictive and denotes copolymers prepared by a copolymerization process following statistical laws (e.g., a chain-growth process). Of course, the above definition can be loosely interpreted. Similarly to alternating or periodic copolymers, some sequence defects and light chain-to-chain sequence deviations can be tolerated (e.g., a copolymer containing 90% of alternating dyads is generally still considered to be an alternating copolymer). Thus, different types of copolymers can be described as aperiodic (Figure 2). It should be noted that the term aperiodic copolymer has already been employed in a few publications.12−15 However, previous uses of this term are not always matching the above definition.
Figure 2.
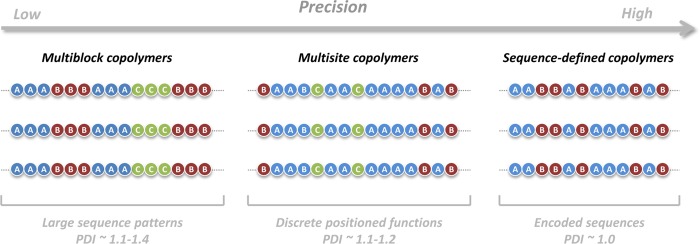
Schematic representation of different types of aperiodic copolymers: multiblock copolymers (left), multisite copolymers obtained using time-controlled monomer additions (middle), and monodisperse sequence-defined copolymers (right). The ellipse points displayed at the extremities only mean that the sequences can be longer than shown.
The simplest example of an aperiodic copolymer is probably a multiblock copolymer, in which the block sequence does not follow a regular pattern (Figure 2, left). It should be reminded that block copolymers have been studied for decades and have therefore their own terminology. For instance, the terms symmetric/asymmetric are often used to denote them. These words, however, do not mean the same thing as periodic/aperiodic. Indeed, the symmetry of a block copolymer depends on block sequence and block length (i.e., on the volume fraction of each block).16−19 For instance, multiblock copolymers composed of repeated AB motifs can be symmetric or asymmetric. The terms periodic and aperiodic allow a simplified description of a linear multiblock sequence. In this case, each block is considered as a unit in the sequence, independently of its length.20 For instance a multiblock ABCABCABC can be described as periodic, i.e., (ABC)3. On the other hand, the block copolymer ABABCBCAC can be named aperiodic. In a recent review, Bates et al. have highlighted the interesting opportunities that could be explored with complex multiblock copolymers.21 However, such structures were for a long time not available because it was difficult to synthesize block copolymers longer than pentablocks using stepwise strategies. Thus, early examples of decablock, undecablock, or dodecablock copolymers reported in the literature were in general periodic structures.22−25 This situation has changed during the past few years with the development of optimal living polymerization techniques for block synthesis. The first example was reported by Coates and co-workers who described the synthesis of aperiodic functional hexablock copolymers.26 Following that example, Whittaker and co-workers have described the synthesis of aperiodic hexablock and octablock copolymers by single-electron transfer living radical polymerization (SET-LRP).27,28 More recently, Perrier and co-workers have reported comparable results using reversible addition–fragmentation chain transfer (RAFT) polymerization.29 Although the chain-end fidelity of SET-LRP and RAFT has been recently questioned,30,31 these examples show that long aperiodic block sequences are now reachable. In this new context, a specific terminology for block sequences would be probably helpful. For example, recently reported icosablock copolymers are difficult to describe using standard naming conventions.29
Another type of aperiodic copolymer is a macromolecule containing discrete functional sites distributed aperiodically along its linear backbone (Figure 2, middle). In such a case, the backbone is predominantly composed of one type of comonomer (i.e., monomer A in the example). Functional sites composed of one or a few monomer units are positioned at specific locations along this backbone (e.g., comonomers B and C in the example of Figure 2). The terms periodic and aperiodic can be used to describe the distribution of these sites along the backbone. However, only the periodicity/aperiodicity of sites B and C is considered (i.e., A is not taken into account). For example, a copolymer containing a repeating site B or alternating sites B and C can be described as periodic.32 On the other hand the structure displayed in the middle of Figure 2 can be named aperiodic. Such multisite copolymers can be obtained by applying external alterations during living polymerizations.33 For instance using time-controlled monomer feeds, a statistical copolymerization process can be intentionally guided to form complex aperiodic sequences.34 The first example of this was reported by our group some years ago.35,36 In this approach, small amounts of functional N-substituted maleimides are added at specific times during the atom transfer radical polymerization (ATRP) of a large excess of styrene. Due to the strong alternating tendency of these monomers with styrene, small functional regions are created at desired locations in the polystyrene backbone. Using a robotic platform, we have recently shown that complex aperiodic codes can be encrypted in the chains.37 Still, this method utilizes a chain-growth polymerization process, and therefore the formed copolymers exhibit sequence defects as summarized in a recent account.38 Nevertheless, we have recently described a concept for minimizing these defects and obtaining ultraprecise sequences.39 Very recently, O’Reilly and co-workers have also reported a related strategy that exploits reactivity differences in ring-opening metathesis polymerization.40 This approach was used to prepare aperiodic microstructures.
As shown on the right side of Figure 2, the next level of complexity is the synthesis of monodisperse sequence-defined copolymers containing aperiodic sequences. Although it is not an official terminology, it was recently proposed that the terms “sequence-controlled” and “sequence-defined” have different meanings (Figure 1a).9 “Sequence-controlled” is a broad generic term that includes all type of polymers with controlled sequence distributions,6 whereas “sequence-defined” refers to copolymers that have perfectly defined primary structures (ribosome-made proteins or resin-made oligomers for example). For instance, alternating copolymers prepared by free-radical polymerization are sequence-controlled but are not sequence-defined. On the other hand, sequence-defined copolymers are a particular subclass of sequence-controlled copolymers as shown in Figure 1a. The most reliable approach for preparing sequence-defined copolymers is iterative solid-phase chemistry, even though interesting alternative concepts have been reported lately.41−43 During the last two decades, solid-phase chemistry has been used to prepare non-natural sequence-defined oligomers.44,45 For instance, peptoids, developed by Zuckermann and co-workers, can be prepared using a wide variety of functional submonomers and in the absence of main-chain protecting groups.46 This interesting chemistry has been studied to build aperiodic polymer structures and related materials.47 During the last years, other interesting protecting-group-free solid-phase approaches have been reported.48−50 For example, our group has described a chemoselective approach using successive copper-catalyzed azide–alkyne cycloaddition (CuAAC) and amidification steps.48 It was recently shown that this strategy can be used to prepare information-containing macromolecules.51 Using three monomers (one spacer and two coding units), it was possible to implement intentionally a binary code in the formed oligomer chains. Although created with a restricted set of monomers, these copolymers are not alternating, not periodic, not gradient, not block, and certainly not random. They are the first example of synthetic macromolecules containing a binary code encoded by aperiodic comonomer sequences. As discussed in a recent essay,10 such macromolecules are promising for encoding information at the molecular level.
In conclusion, the aim of the present viewpoint was to describe a new class of copolymers. Aperiodic copolymers are sequence-controlled copolymers that are not composed of repeating sequence motifs. As shown herein, numerous examples of aperiodic copolymers have been reported in recent literature. In this context, it is certainly time to revise the terminology of copolymers. Polymer chemists are now creating highly complex copolymer structures, which are not depicted in textbooks and official glossaries. For instance, classical microstructures such as gradient copolymers (Figure 1b),52 which have been extensively studied for about 15 years, are still not included in the official nomenclature of polymers.5 It would be probably very beneficial for our community if the debate on copolymer terminology could be reopened. Besides pure terminology considerations, it should be clearly stated that new types of copolymer such as aperiodic copolymers may open a world of new opportunities for conceiving polymer materials. Indeed, only aperiodicity allows creation of complex symphonies and languages.
Acknowledgments
This work was supported by the European Research Council (ERC Grant agreement 258593) and the CNRS. JFL thanks the University of Strasbourg, the Excellence Center for Complex Chemical Systems (LabEx CSC), and the International Center for Frontier Research in Chemistry (icFRC) for their support. Ron Zuckermann (Lawrence Berkeley National Laboratory) is also greatly acknowledged for his helpful comments and suggestions.
The authors declare no competing financial interest.
References
- Schrödinger E.What is Life?; The McMillan Company: New York, 1945. [Google Scholar]
- Watson J. D.; Crick F. H. C. Nature 1953, 171, 737. [DOI] [PubMed] [Google Scholar]
- It should be noted that long-range correlations have been observed in nucleotide sequences. Although this topic is out of the scope of this viewpoint, additional information can be found in the following article:Peng C. K.; Buldyrev S. V.; Goldberger A. L.; Havlin S.; Sciortino F.; Simons M.; Stanley H. E. Nature 1992, 356, 168. [DOI] [PubMed] [Google Scholar]
- Ring W.; Mita I.; Jenkins A. D.; Bikales N. M. Pure Appl. Chem. 1985, 57, 1427. [Google Scholar]
- Compendium of Polymer Terminology and Nomenclature, IUPAC Recommendations 2008, 2nd ed.; Jones R. G., Wilks E. S., Metanomski W. V., Kahovec J., Hess M., Stepto R., Kitayama T., Eds.; RSC: U.K., 2009. [Google Scholar]
- Lutz J.-F.; Ouchi M.; Liu D. R.; Sawamoto M. Science 2013, 341, 1238149. [DOI] [PubMed] [Google Scholar]
- Badi N.; Lutz J.-F. Chem. Soc. Rev. 2009, 38, 3383. [DOI] [PubMed] [Google Scholar]
- Lutz J.-F. Polym. Chem. 2010, 1, 55. [Google Scholar]
- Sequence-Controlled Polymers: Synthesis, Self-Assembly, and Properties; ACS Symposium Series; Lutz J.-F., Meyer T., Ouchi M., Sawamoto M., Eds.; ACS: Washington DC, 2014, Vol. 1170. [Google Scholar]
- Colquhoun H.; Lutz J.-F. Nat. Chem. 2014, 6, 455. [DOI] [PubMed] [Google Scholar]
- The IUPAC Gold book includes the definition of an irregular macromolecule. The words irregular and aperiodic have related meanings. However, the current definition of an irregular macromolecule is broad and includes uncontrolled situations (e.g., random copolymers). There is also no official definition for an irregular copolymer.
- Blackwell J.; Gutierrez G. A.; Chivers R. A. Macromolecules 1984, 17, 1219. [Google Scholar]
- Stupp S. I.; Moore J. S.; Martin P. G. Macromolecules 1988, 21, 1228. [Google Scholar]
- Stupp S. I.; Wu J. L.; Moore J. S.; Martin P. G. Macromolecules 1991, 24, 6399. [Google Scholar]
- Bakhshi A. K.; Pooja J. Chem. Soc., Faraday Trans. 1996, 92, 5017. [Google Scholar]
- Leibler L. Macromolecules 1980, 13, 1602. [Google Scholar]
- Bates F. S. Science 1991, 251, 898. [DOI] [PubMed] [Google Scholar]
- Matsen M. W.; Bates F. S. Macromolecules 1996, 29, 1091. [Google Scholar]
- Ruzette A.-V.; Leibler L. Nat. Mater. 2005, 4, 19. [DOI] [PubMed] [Google Scholar]
- It should be reminded to nonspecialist readers that the letters ABC are generally used to denote a single block in a block copolymer; i.e., the letter A denotes a series of similar monomers -AAAAAAAA-. For instance, the multiblock copolymer shown on the left of Figure 2 would be named ABACB. This should not be confused with the ABC code that is employed to describe random, alternating, and periodic copolymers. In such copolymers, each capital letter denotes a single monomer unit.
- Bates F. S.; Hillmyer M. A.; Lodge T. P.; Bates C. M.; Delaney K. T.; Fredrickson G. H. Science 2012, 336, 434. [DOI] [PubMed] [Google Scholar]
- Koo C. M.; Hillmyer M. A.; Bates F. S. Macromolecules 2005, 39, 667. [Google Scholar]
- Nagata Y.; Masuda J.; Noro A.; Cho D.; Takano A.; Matsushita Y. Macromolecules 2005, 38, 10220. [Google Scholar]
- Wu L.; Lodge T. P.; Bates F. S. Macromolecules 2005, 39, 294. [Google Scholar]
- Sugiyama K.; Oie T.; El-Magd A. A.; Hirao A. Macromolecules 2010, 43, 1403. [Google Scholar]
- Kim J. G.; Cowman C. D.; LaPointe A. M.; Wiesner U.; Coates G. W. Macromolecules 2011, 44, 1110. [Google Scholar]
- Soeriyadi A. H.; Boyer C.; Nyström F.; Zetterlund P. B.; Whittaker M. R. J. Am. Chem. Soc. 2011, 133, 11128. [DOI] [PubMed] [Google Scholar]
- Boyer C.; Soeriyadi A. H.; Zetterlund P. B.; Whittaker M. R. Macromolecules 2011, 44, 8028. [Google Scholar]
- Gody G.; Maschmeyer T.; Zetterlund P. B.; Perrier S. Nat. Commun. 2013, 4. [DOI] [PubMed] [Google Scholar]
- Harrisson S.; Nicolas J. ACS Macro Lett. 2014, 3, 643. [DOI] [PubMed] [Google Scholar]
- Vandenbergh J.; Junkers T. Macromolecules 2014, 475051. [Google Scholar]
- Berthet M.-A.; Zarafshani Z.; Pfeifer S.; Lutz J.-F. Macromolecules 2010, 43, 44. [Google Scholar]
- Leibfarth F. A.; Mattson K. M.; Fors B. P.; Collins H. A.; Hawker C. J. Angew. Chem., Int. Ed. 2013, 52, 199. [DOI] [PubMed] [Google Scholar]
- Lutz J.-F.; Schmidt B. V. K. J.; Pfeifer S. Macromol. Rapid Commun. 2011, 32, 127. [DOI] [PubMed] [Google Scholar]
- Pfeifer S.; Lutz J.-F. J. Am. Chem. Soc. 2007, 129, 9542. [DOI] [PubMed] [Google Scholar]
- Pfeifer S.; Lutz J.-F. Chem.—Eur. J. 2008, 14, 10949. [DOI] [PubMed] [Google Scholar]
- Chan-Seng D.; Zamfir M.; Lutz J.-F. Angew. Chem., Int. Ed. 2012, 51, 12254. [DOI] [PubMed] [Google Scholar]
- Lutz J.-F. Acc. Chem. Res. 2013, 46, 2696. [DOI] [PubMed] [Google Scholar]
- Zamfir M.; Lutz J.-F. Nat. Commun. 2012, 3, 1138. [Google Scholar]
- Moatsou D.; Hansell C. F.; O’Reilly R. K. Chem. Sci. 2014, 5, 2246. [Google Scholar]
- McKee M. L.; Milnes P. J.; Bath J.; Stulz E.; Turberfield A. J.; O’Reilly R. K. Angew. Chem., Int. Ed. 2010, 49, 7948. [DOI] [PubMed] [Google Scholar]
- Lewandowski B.; De Bo G.; Ward J. W.; Papmeyer M.; Kuschel S.; Aldegunde M. J.; Gramlich P. M. E.; Heckmann D.; Goldup S. M.; D’Souza D. M.; Fernandes A. E.; Leigh D. A. Science 2013, 339, 189. [DOI] [PubMed] [Google Scholar]
- Niu J.; Hili R.; Liu D. R. Nat. Chem. 2013, 5, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C. Y.; Moran E. J.; Cherry; Stephans J. C.; Fodor S. P.; Adams C. L.; Sundaram A.; Jacobs J. W.; Schultz P. G. Science 1993, 261, 1303. [DOI] [PubMed] [Google Scholar]
- Hartmann L.; Börner H. G. Adv. Mater. 2009, 21, 3425. [DOI] [PubMed] [Google Scholar]
- Sun J.; Zuckermann R. N. ACS Nano 2013, 7, 4715. [DOI] [PubMed] [Google Scholar]
- Rosales A. M.; Segalman R. A.; Zuckermann R. N. Soft Matter 2013, 9, 8400. [Google Scholar]
- Pfeifer S.; Zarafshani Z.; Badi N.; Lutz J.-F. J. Am. Chem. Soc. 2009, 131, 9195. [DOI] [PubMed] [Google Scholar]
- Espeel P.; Carrette L. L. G.; Bury K.; Capenberghs S.; Martins J. C.; Du Prez F. E.; Madder A. Angew. Chem., Int. Ed. 2013, 52, 13261. [DOI] [PubMed] [Google Scholar]
- Solleder S. C.; Meier M. A. R. Angew. Chem., Int. Ed. 2014, 53, 711. [DOI] [PubMed] [Google Scholar]
- Trinh T. T.; Oswald L.; Chan-Seng D.; Lutz J.-F. Macromol. Rapid Commun. 2014, 35, 141. [DOI] [PubMed] [Google Scholar]
- Matyjaszewski K.; Ziegler M. J.; Arehart S. V.; Greszta D.; Pakula T. J. Phys. Org. Chem. 2000, 13, 775. [Google Scholar]


