Abstract
Aim
To study the efficacy of novel rhenium compounds to treat triple node negative breast cancer.
Place and Duration
Six (6) novel rhenium pentycarbanato compounds (PC1-6) were synthesized and triple node negative breast cancer cell lines HTB-132 and Balb/c mouse kidney cell lines were treated with each of them for 48 hours. The results were analyzed by a common trypan blue cell death assay system and statistically analyzed.
Place and Duration
The compounds were synthesized, analyzed and evaluated at the Department of Chemistryof Morgan State University, Baltimore, Maryland and the Pharmaceutical Sciences Department of Elizabeth City State University campus of the University of North Carolina system.
Methodology
The novel rhenium compounds were synthesized from one-pot reactions of Re2(CO)10 with the corresponding α-diimine ligands in 1-pentanol.The compounds were characterized spectroscopically.
The cell lines were cultured by standard cell culture procedure and treated with each of the six compounds in DMSO for 48 hours with a negative control and a DMSO vehicular control along with a cisplatin positive control.The cytotoxicity was evaluated by standard trypan blue assay and the results were statistically analyzed.
Results
The trypan blueassay reveals that these compounds have significant cytotoxicity against MDA-MB-468 (HTB-132) triple node negative breast cancer cell lines and are less nephrotoxic than cisplatin.
Conclusion
The novel rhenium compounds PC 1-6 can potentially find applications in the treatment of highly malignant triple node negative breast cancer.
Keywords: Rhenium pentylcarbonato compounds, HTB-132 breast cancer cells, cytotoxicity, trypan blue assay
1.INTRODUCTION
Breast cancer is the most common cancer among women.There are several drugs developed to treat ER(+) breast cancers but very few or no drugs are there to treat ER(-) especially triple negative breast cancers. Scientists reported success in treating breast cancer with the organic drugs tamoxifen and raloxifene, shrinking advanced tumors with herceptin and treating early cancers with taxol. However, these drugs have severe side effects. For example, tamoxifen causes human endometrial cancer and liver cancer in rats [1,2]. Numerous inorganic anticancer agents have been synthesized [3-5] and only a few of them have been found to be effective against breast cancer. It has been shown that, in combination with other drugs, cisplatin, cis-[Pt (NH3)2Cl2], may be effective against breast cancer [6]. Also the cisplatin analogs, oxaliplatin and DWA-2114R [7] have been demonstrated to be cytotoxic against breast cancer cells. Cisplatin, carboplatin, oxaliplatin and related metallodrugs are extensively being used in the treatment of a variety of cancers. Unfortunately these drugs are highly toxic and drug-resistance develops over time in cancer cells. These circumstances have led researchers to look for new cytotoxic agents which exhibit less toxicity and no drug resistance. Recently a group of organometallic derivatives of tamoxifen, called ferrocifens have been found to be active against both ER(+) and ER(-) breast cancers [8].
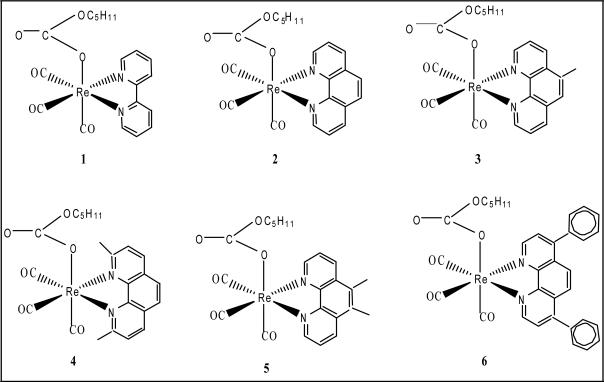
Several rhenium carbonyl complexes have been reported to be cytotoxic against numerous cancer cell lines [9,10]. Many of these complexes have been found to display cytotoxicity against breast cancer cell lines. However, systematic treatments of these drugs with various breast cancer cell lines including MDA breast cancer cell lines have not been reported.Recently we synthesized several novel rhenium pentylcarbonato compounds [11]. The structural formulae of the compounds (PC 1-6) are shown in Fig. 1. Here we report the cytotoxic effects of PC 1-6 against triple node negative breast cancer cell lines MDA-MB-468 (HTB-132) and Balb/c mouse kidney cell lines.
Fig. 1.
The structural formulae of the PC-series compounds PC 1-6
2. MATERIALS AND METHODS
2.1 Materials
MDA-MB-468 (HTB-132) triple node negative breast cancer cell lineswere obtained from ATCC (USA), cultured inL-15 medium supplemented with 10% Fetal Bovine Serum(FBS), penicillin and streptomycin antibiotics and grownat 37°C inan incubator. The mouse glomerular mesangialcell line was a kind gift from Dr. C. Reilly of Virginia Tech University, USA. These cells were cultured in RPMI-1640 medium supplemented with 10%FBS, penicillin and streptomycin antibiotics and grown at 37°C in a carbon dioxide incubator.
Synthesis of rhenium pentacarbanato compounds PC 1-6: The rhenium compounds PC 1-6 were synthesized according to published procedure (Mbagu et. al. 2012). In brief, a mixture of Re2(CO)10 and the corresponding α-diimines in 1:2 mole ratio was refluxed in 1-pentanol while CO2 was bubbled through the solutions. The α-diimines in PC 1–6 are 2,2′-bipyridyl, 1,10-phenanthroline, 5-methyl-1,10-phenanthroline, 2,9-dimethyl-1,10-phenanthroline, 5, 6-dimethyl-1,10-phenanthrolineand4,7-diphenyl-1,10-phenanthroline,respectively. The reaction was discontinued after 24 hours.After cooling to room temperature, the solid pentylcarbonato complexes PC 1–6 were isolated by filtration.
2.2 Cytotoxicity Assay
HTB-132 breast cancer cell lines were grown in six well plates to confluency, the cells were then kept in serum-free medium overnight and then treated with each of drugsPC1-6 (dissolved in DMSO) along with untreated cells and DMSO vehicular control for 48 hours. Since previous studies with different cancer cell lines [11] showed 50% cell death (IC50)dose for these compounds ranged between 2-5 μM, we selected that range as our loading dose. Atrypan blue assay was done to measure cell viability using a standard hemocytometer and light microscope.
2.3 Statistical Analysis
Each experiment was repeated three times and statistical t test was used to analyze the results with P<0.05.
3. RESULTS
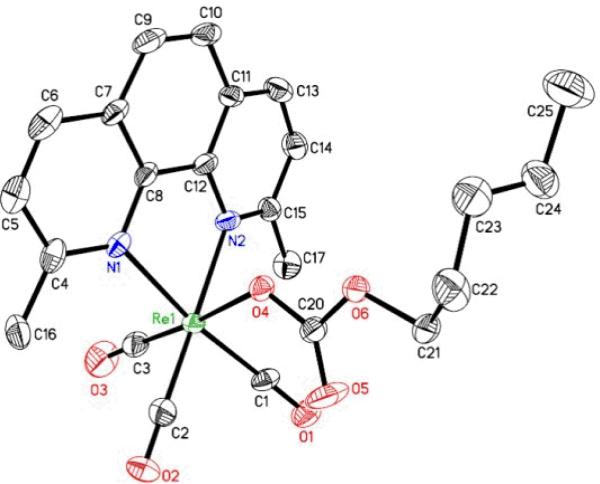
The pentyl carbonato compounds were characterized spectroscopically[11]. Additionally, PC 1, PC 4 and PC 6 were characterized through X-ray crystal structure determinations. The X-ray structures of PC 1 and PC 6 were reported earlier (CCDC 749606 for PC 1 and CCDC 749607 for PC 6 contain the supplementary crystallographic data.These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif). The X-ray structure of PC 4 has been carried out recently and is shown in Fig. 2. The crystallographic data and other related information for PC 4 will be published elsewhere.
Fig. 2.
X-ray structure of PC 4
Table 1 shows the effects of PC 1-6 on MDA-MB-468 (HTB-132) and Balb/c mouse kidney cell lines. All the drugs were cytotoxic to the breast cancer cell lines and found to be less nephrotoxic thancisplatin.
Table 1.
The effects of PC 1-6 on MDA-MB-468 (HTB-132) and Balb/c mouse kidney cell lines
| Drugs | Breast Cancer HTB-132 cell lines | Mouse Mesangial cell lines |
|---|---|---|
| RPC1 | 3±2.5 | 5±2.5 |
| RPC2 | 2±1.5 | 5±3.5 |
| RPC3 | 3±2.5 | 4±2.5 |
| RPC4 | 2±2.5 | 5±5.5 |
| RPC5 | 3±4.5 | 4±2.5 |
| RPC6 | 4±6.5 | 5±1.5 |
| Cisplatin | 5±2.5 | 1±2.5 |
IC- 50 (mean ± S.D.) refers to the concentration required to have 50% cell-growth inhibition; DMSO was inactive in both the cell lines tested.
3. DISCUSSION
Anticancer drugs follow a variety of modes (mechanisms) of action.Cisplatin and related compounds produce intrastrand d(GpG) and d(ApG) adducts [12]. Dinuclear platinum complexes produce DNA (Pt,Pt) interstrand and intrastrand crosslinks [13]. The tetranuclear platinum thiosemicarbazone complex forms DNA interhelical crosslinks [14]. A selective transport mechanism into the cell via transferrin was proposed for ruthenium imidazole complexes [15,16]. The behavior of organometallic metallocenes with DNA is very different to that of cisplatin [17]. Cisplatin and related drugs directly bind (form covalent bond) to the DNA.As a result normal cells specially kidney cells are highly affected resulting in severe toxic side effects.In this study, we found the PC compounds to be effective anticancer agents with less nephrotoxicity than cisplatin.
Almost all the PC-series compounds (drugs) showed effective cytotoxicity against the triple node negative breast cancer cells for 48 hours exposure.
Triple node negative breast cancer is a fulminant radio-resistant malignant tumor that has almost no known drugs that is an effective chemotherapeutic agent. Further studies are needed with more different node negative breast cancer cell lines and eventually in animal models to come to a conclusion about the real effectiveness of these drugs.Our research demonstrates that the rhenium compounds are effective against triple node negative breast cancer cells. Further studies need to be done regarding the mechanism, nature of cell death and pathway involved in induction of cell death by these compounds.
ACKNOWLEDGEMENTS
Partially supported by US-NIH-RISE, US-National Science Foundation LSAMP institutional support Award to Elizabeth City State University, NC, TMCF-DOE award to Dr. H. Banerjee and the work at MSU was partially supported by grants from the National Institutes of Health (Grant No.G11HD038439) and Nuclear Regulatory Commission (Grant No. NRC-HQ-12-G-27-0086). Crystallographic data were collected through the SCrALS (Service Crystallography at Advanced Light Source) program at Beamline 11.3.1 at the Advanced Light Source (ALS), Lawrence Berkeley National Laboratory. The ALS is supported by the U.S. Department of Energy, Office of Energy Sciences Materials Sciences Division, under contract DE-AC02-05CH11231.
Footnotes
Authors’ contributions
This work was carried out in collaboration between all authors. Author HNB shaped the first and second drafts of the manuscript and did all the corrections mentioned by the reviewers. Author DG synthesized the compounds. Author AW helped characterize the compounds. Authors CP, VS and CK studied the anticancer properties, treated the kidney cells and performedthe statistical analysis. Author CR provided andhelped study thekidney cells. Author JAK solved the molecular structureof the compound PC4. Author JMW managed literature searches. Author SKM wrote the final manuscript and did the corrections suggested by the Editorial Office. All authors read and approved the final manuscript.
COMPETING INTERESTS
Authors have declared that no competing interests exist.
REFERENCES
- 1.Fisher B, Constantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WL. Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14.J. Natl. Cancer Inst. 1994;86:527–37. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 2.Davis W, Venitt S, Phillips DH. The metabolic activation of α-hydroxytamoxifen to DNA-binding species in rat tamoxifen and hepatocytes proceeds via sulphation. Carcinogenesis. 1998;19:861–66. doi: 10.1093/carcin/19.5.861. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann M, Keppler BK. Inorganic anticancer agents: Their chemistry and antitumor properties. Comments Inorg. Chem. 1995;16(6):339–72. [Google Scholar]
- 4.Keppler BK, Hartmann M. New tumor-inhibiting metal complexes. Chemistry and antitumor properties. Met.-Based Drugs. 1994;1(2-3):145–49. doi: 10.1155/MBD.1994.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahrovic E. Chemical feature of inorganic compounds as anticancer agents. Health Med. 2011;5(5):1112–16. [Google Scholar]
- 6.Pil P, Lippard SJ. Cisplatin and related drugs. Encyclopedia of Cancer. 1997;1:392–410. [Google Scholar]
- 7.Majima H. In: Platinum and Other Metal Coordination Compounds in Cancer Chemotherapy. Howell SB, editor. Plenum Press; New York: 1991. pp. 345–55. [Google Scholar]
- 8.Dallagi T, Saidi M, Vessières A, Huché M, Jaouen G, Top S. Synthesis and antiproliferative evaluation of ferrocenyl and cymantrenyl triaryl butene on breast cancer cells. Biodistribution study of the corresponding technetium-99m tamoxifen conjugate. J. Organomet. Chem. 2013;734:69–77. [Google Scholar]
- 9.Wang W, Yan YK, Hor TSA, Vittal JJ, Wheaton JR, Hall IH. Synthesis, X-ray structures and cytotoxicity of rhenium(I) carbonyl 2-(dimethylamino) ethoxidecomplexes. J. Organomet. Chem. 2002;21:1991–99. [Google Scholar]
- 10.Yan YK, Cho SE, Shaffer KA, Rowell JE, Barnes BJ, Hall IH. Cytotoxicity of rhenium(I) alkoxo and hydroxocarbonyl complexes in murine and human tumor cells. Pharmazie. 2000;55:307–13. [PubMed] [Google Scholar]
- 11.Mbagu MK, Kebulu ND, Winstead A, Pramanik SK, Banerjee HN, Iwunze MO, Wachira JM, Greco GE, Haynes G, Sehmer A, Sarkar FH, Ho DM, Pike RD, Mandal SK. Factricarbonyl(pentylcarbonato)(α-diimine)rhenium complexes: One-potsynthesis, characterization, fluorescence studies, and cytotoxic activity against human MDA-MB-231 breast, CCl-227 colon and BxPC-3 pancreatic carcinoma cell lines. Inorg. Chem. Comm. 2012;21:35–38. [Google Scholar]
- 12.Umapathy P. The chemical and biochemical consequences of the binding of the antitumor drug cisplatin and other platinum metal complexes to DNA. Coord. Chem. Rev. 1989;95:129–81. [Google Scholar]
- 13.Farrell N. DNA binding and chemistry of dinuclearplatinum complexes. Comments Inorg. Chem. 1995;16(6):373–89. [Google Scholar]
- 14.Quiroga AG, Perez JM, Lopez-Solera I, Masaguer JR, Luque A, Roman P, Edwards A, Alonso C, Navarro-Ranninger C. Novel tetranuclearorthometallatedcomplexes of Pd(II) and Pt(II) derived from p-isopropylbenzaldehydethiosemicarbazone with cytotoxic activity in cis-DDP resistant tumor cell lines. Interaction of these complexes with DNA. J. Med. Chem. 1998;41:1399–1408. doi: 10.1021/jm970520d. [DOI] [PubMed] [Google Scholar]
- 15.Kratz F, Hartmann M, Keppler B, Messori L. The binding properties of two antitumor ruthenium(III) complexes to apotransferrin. J. Biol. Chem. 1994;269(4):2581–88. [PubMed] [Google Scholar]
- 16.Kratz F, Keppler BK, Hartmann M, Messori L, Berger MR. Comparison of the antiproliferative activity of two antitumor ruthenium(III) complexes with their apotransferrin and transferrin-bound forms in a human colon cancer cell line. Met.-Based Drugs. 1996;3(1):15–23. doi: 10.1155/MBD.1996.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo LY, Kanatzidis MG, Sabat M, Tipton AL, Marks TJ. Metalloceneantitumor agents. Solution and solid-state molybdenocenecoordination chemistry of DNA constituents. J. Am. Chem. Soc. 1991;113:9027–45. [Google Scholar]