Abstract
Patients with cystic fibrosis (CF) often suffer chronic lung infection with concomitant inflammation, a setting that may reduce the efficacy of gene transfer. While gene therapy development for CF often involves viral-based vectors, little is known about gene transfer in the context of an infected airway. In this study, three mouse models were established to evaluate adeno-associated virus (AAV) gene transfer in such an environment. Bordetella bronchiseptica RB50 was used in a chronic, nonlethal respiratory infection in C57BL/6 mice. An inoculum of ∼105 CFU allowed B. bronchiseptica RB50 to persist in the upper and lower respiratory tracts for at least 21 days. In this infection model, administration of an AAV vector on day 2 resulted in 2.8-fold reduction of reporter gene expression compared with that observed in uninfected controls. Postponement of AAV administration to day 14 resulted in an even greater (eightfold) reduction of reporter gene expression, when compared with uninfected controls. In another infection model, Pseudomonas aeruginosa PAO1 was used to infect surfactant protein D (SP-D) or surfactant protein A (SP-A) knockout (KO) mice. With an inoculum of ∼105 CFU, infection persisted for 2 days in the nasal cavity of either mouse model. Reporter gene expression was approximately ∼2.5-fold lower compared with uninfected mice. In the SP-D KO model, postponement of AAV administration to day 9 postinfection resulted in only a two fold reduction in reporter gene expression, when compared with expression seen in uninfected controls. These results confirm that respiratory infections, both ongoing and recently resolved, decrease the efficacy of AAV-mediated gene transfer.
Introduction
Cystic fibrosis (CF) is the most common lethal genetic disorder among Caucasians, occurring at a rate of approximately 1 in 2000 people (Bobadilla et al., 2002). As a disease caused by a single defective gene, CF airway disease is ideal for treatment with gene therapy (Lee et al., 2005). However, over 20 years have elapsed since the discovery of the CF transmembrane conductance regulator and the associated CFTR gene, and still, no viable gene therapy option exists for curing or alleviating airway disease (Birault et al., 2013). A number of obstacles to gene therapy for CF exist (Ferrari et al., 2002; Oakland et al., 2012), but one major obstacle has yet to be extensively studied: how an active respiratory infection affects gene transfer in the airway.
Patients with CF are characterized by chronic respiratory infections by opportunistic bacteria (Chattoraj et al., 2010). In particular, Pseudomonas aeruginosa colonizes the lungs of most CF patients indefinitely (Govan and Deretic, 1996). The initial presentation of infection is not fatal, but as CF patients cannot clear the bacteria from their lungs, a persistent inflammatory state arises that causes continuous infection and eventually leads to permanent pulmonary damage (Sinn et al., 2011). Little research has been done to investigate the effects of microbial infection on airway gene transfer.
Deterioration of the lung in CF patients is caused by a cycle of infection by opportunistic pathogens and neutrophilic inflammation (Makam et al., 2009). By the age of 17, nearly 70% of CF patients have P. aeruginosa in their sputum at levels of 106–108 CFU/gr sputum (Davis et al., 1996), and approximately 80% of adult CF patients are chronically infected with P. aeruginosa that cannot be eliminated by antibiotic therapy (Cystic Fibrosis Foundation, 2012). In a phase II gene therapy trial with adeno-associated virus (AAV)-CFTR, nearly 70% of CF patients receiving the AAV2 vector were P. aeruginosa colonized (Moss et al., 2004). The presence of infection was an endpoint of measurement in the trial and was not a basis for exclusion from the trial. Separately, a phase IIB trial with AAV2-CFTR that included 3 doses of 1013 particles administered 30 days apart failed to demonstrate efficacy (Moss et al., 2007). In this clinical trial of 102 CF subjects, the number of days of antibiotic use was considered a marker for trial monitoring. Infection in CF airways is expected during the progress of the gene therapy regimen, and about 15% of CF subjects were on antibiotics while they received aerosolized AAV2 for this study.
In a clinical trial of interferon gamma-1b with 66 CF patients, sputum bacterial density was 7.1 Log10 CFU/gr, with 80–90% of enrolled subjects having P. aeruginosa infection (Moss et al., 2005). The sickest CF patients have chronic lung infection, but the presence of P. aeruginosa in sputum has not been a criterion for exclusion in airway-directed gene therapy clinical trials. The failure of CF AAV gene therapy trials may be due in part to the difficulty of gene delivery to lungs that sustain and harbor a chronic disease state because of infection.
The paucity of research in this area may be related to the lack of a relevant animal model necessary to study such effects. CFTR knockout (KO) mice do not develop the pulmonary abnormalities typically associated with the lung disease in humans (Pezzulo et al., 2012). Additionally, P. aeruginosa is not a natural murine pathogen and cannot naturally establish a lasting infection in wild-type (WT) mice, unless administered in an artificial biofilm (i.e., embedded in beads composed of agar, agarose, or seaweed alginate) (Hoffmann et al., 2005). Administration of free bacteria results in either rapid clearance of the organism or acute sepsis and death (van Heeckeren and Schluchter, 2002).
We present here three alternatives for studying gene transfer in an infected airway. The first involves a natural murine pathogen, Bordetella bronchiseptica (Bb) RB50, to establish a chronic infection in C57BL/6 mice. Bb infects a number of members of the mammalian family, and in rodents, rabbits, cats, dogs, and pigs, it typically establishes an asymptomatic infection in the nasal cavity that persists indefinitely (Gueirard et al., 2003; Irie and Yuk, 2007). In the lower respiratory tract, the infection is enduring, but transient, with the bacterial load reducing to almost baseline within 45 days (Gueirard et al., 2003). The second and third scenarios utilize P. aeruginosa PAO1 to establish an acute infection in surfactant protein D (SP-D) and surfactant protein A (SP-A) KO mice, respectively. SP-D and SP-A are pulmonary collectins that are important in innate immunity against various bacterial and viral pathogens (Cheng and Palaniyar, 2013). Decreased or lack of SP-D and SP-A has been implicated in the pathogenesis of CF airway disease (Postle et al., 1999). When KO mice were engineered to lack either SP-D or SP-A, they were found to have decreased ability to effectively clear P. aeruginosa (Giannoni et al., 2006). The resulting infection was still transient, lasting only a few days, but the resulting inflammatory response was exaggerated when compared with WT mice. As hyperinflammatory responses are also typical of CF patients, the SP-D and SP-A KO mice emulate key inflammatory aspects of the disease when compared with WT mice.
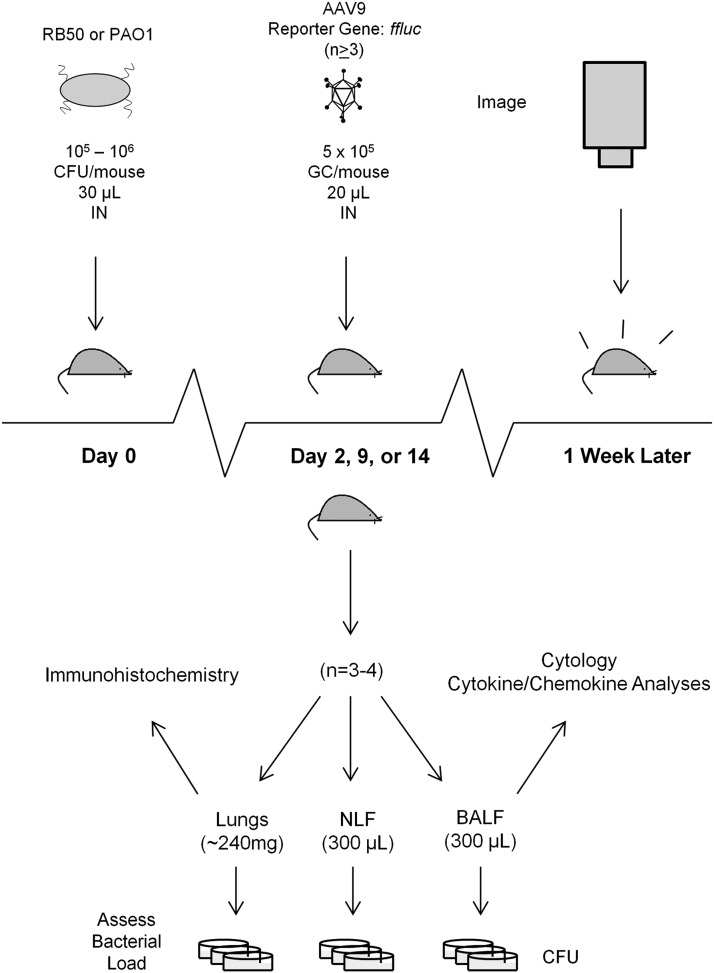
In this report, we employed these mouse models to study AAV-mediated gene transfer in infected airways. Figure 1 depicts the experimental design used. The presence of an on-going or even recently resolved infection decreased AAV-mediated gene transfer efficiency in the airway, validating the use of these models to optimize gene transfer for relevant airway diseases like CF.
FIG. 1.
Schematic of experimental design. Mice are i.n. infected with freshly cultured bacteria on day 0. On day 2, 9, or 14, an AAV9 vector containing a ffluc reporter gene is introduced to the recently infected airway via intranasal instillation. Three to four randomly selected mice are also taken on the day of AAV administration for evaluation of bacterial load and characterization of immune response at the time of vector administration (n=3 for uninfected controls, n=4 for infected groups). Approximately every week thereafter, the remaining mice are dosed with luciferin and subjected to live imaging to evaluate ffluc expression. AAV, adeno-associated virus; BALF, bronchoalveolar lavage fluid; ffluc, firefly luciferase; i.n., intranasally; NLF, nasal lavage fluid.
Materials and Methods
Bacterial strains and growth conditions
Bb strain RB50 was a kind gift from Dr. Yasuhiko Irie, University of Washington, Seattle, WA. Bb was cultured in Stainer–Scholte broth or on Bendet Gengou (BG) blood agar (BD Diagnostic Systems) at 37°C. P. aeruginosa PAO1 was a kind gift from Dr. Robert Bucki, University of Pennsylvania, Philadelphia, PA. The bacterium was cultured at 37°C in Miller's Lysogeny Broth (Mediatech) or on Pseudosel Agar (cetrimide agar) from BD Diagnostic Systems.
AAV preparation
The AAV9 vectors flanked with AAV2 inverted terminal repeats contained a firefly luciferase (ffluc) reporter gene fused to a nucleus localization sequence at the N-terminus under the transcriptional control of the cytomegalovirus-enhanced chicken-β-actin promoter. Vectors were produced by the Penn Vector core as previously described (Bell et al., 2011).
Mice
C57BL/6 male mice were purchased from Charles River Laboratories, while SP-D and SP-A KO mice (Botas et al., 1998) were maintained in-house at the University of Pennsylvania. Mice were age-matched and used between 7 and 14 weeks of age. For all experiments, a group size of at least three mice was used for each experimental cohort. All animals were maintained at the Animal Facility of the Translation Research Laboratories at the University of Pennsylvania under protocols reviewed and approved by the University of Pennsylvania's Institutional Animal Care and Use Committee. Before all intranasal administrations, mice were anesthetized by an intraperitoneal injection of ketamine/xylazine (70/7 mg/kg) and were subsequently suspended from their dorsal incisors (hind quarters supported) for dosing.
Preparation of inoculum
Bacteria were grown overnight in their appropriate liquid media at 37°C with shaking at 250 rpm. Approximately 16–18 hr later, the bacteria were diluted into fresh broth to an optical density (λ=600 nm) reading of 0.1 and were allowed to grow until midlogarithmic phase (optical density at λ=600 nm of ∼0.5 for Bb RB50 and ∼0.4 for P. aeruginosa). At that point, the bacteria were harvested and washed once in phosphate-buffered saline (PBS) before being diluted into fresh PBS at the desired concentration (controlled by evaluation of optical density at 600 nm).
Mouse infection model for evaluation of airway transduction
Before challenge, mice were anesthetized intraperitoneally and suspended from their dorsal incisors, as described earlier. Mice were then intranasally (i.n.) challenged with a 30 μl bolus (delivered as two 15 μl aliquots, one into each nostril) of Bb RB50 or P. aeruginosa PAO1 to achieve approximately 105 CFU/mouse. At specified time points later (i.e., 2, 9, or 14 days after infection), mice were i.n. administered 5×1010 genome copies of AAV vector in 20 μl (10 μl into each nostril).
To evaluate ffluc expression, mice (∼20 g) were anesthetized and suspended before 20 μl of D-luciferin (15 mg/ml) was i.n. administered as two 10 μl aliquots, one into each nostril. After 5 min, mice were imaged for 60 sec with the IVIS Xenogen imaging system. Quantification of signal was calculated with Living Image 2.5.1 software.
Evaluation of bacterial load
To determine bacterial load, three samples were taken from each randomly selected mouse: (a) lungs, (b) bronchoalveolar lavage fluid (BALF), and (c) nasal lavage fluid (NLF). Before the lungs were harvested, BALF was collected by instilling 500 μl of sterile PBS through a cannula into the trachea. Fluid was collected and re-instilled into the lungs for a total of three times before being collected into a 1.5 ml tube. The lungs (∼110–150 mg per lung) were then excised. One lung was inflated with a 1:1 mixture of Tissue Tek OCT and PBS, embedded in OCT in a storage cassette and flash-frozen with cooled 2-methylbutane. Frozen lungs were kept at −80°C until further histological processing. The other lung was homogenized in 1.5 ml of sterile PBS for evaluation of bacterial load. For the NLF samples, mice were decapitated and a cannula attached to a 1 ml syringe containing 300 μl of sterile PBS was placed into the tracheal remnant. PBS was then flushed through the nasal passages and collected through the nares into a 1.5 ml tube. This recovered fluid was used to flush the nasal cavity another two times, for a total of three flushes. All samples were kept on ice until further processing.
To determine the CFU counts in each sample, solutions were diluted 10-, 100-, and 1000-fold. Ten-microliter aliquots of each dilution were spotted in duplicate on the appropriate solid selection agar for each bacterial strain (BG blood agar for Bb and Pseudosel agar for P. aeruginosa). Plates were incubated at 37°C for 48 hr for Bb RB50 and 16 hr for P. aeruginosa. The number of colonies at each dilution was then counted, and the concentration of CFU of each sample was back calculated from the dilution factor.
BALF cell analysis
Cytospin slides were prepared using 50 μl of freshly isolated BALF diluted into 100 μl of fresh PBS. After centrifugation (Shandon Cytospin 3; Thermo Fisher Scientific) for 5 min at 1,000 rpm, cells were allowed to air-dry for 10 min. Cells were then fixed with 10% neutral buffered formalin, washed in PBS, stained with Nuclear Fast Red (Sigma Aldrich), dehydrated with a series of ethanols, and cleared with xylene according to standard protocols. Cells were studied and characterized using light microscopy (IX81; Olympus America).
Lung immunohistochemistry
Immunofluorescence staining was performed on frozen lung sections. Sections were fixed in acetone at −20°C for 7 min, air-dried, blocked in 1% donkey serum in PBS for 20 min, and incubated with primary rat antibodies diluted in blocking buffer against CD8 (clone 53–6.7; BD Biosciences; 1:20), CD4 (clone RM4-5; BD Biosciences; 1:20), and Mac-2 (clone M3/38; Cedarlane; 1:200) for 45 min. After washing in PBS, sections were stained with secondary donkey antibodies labeled with FITC or TRITC (Jackson Immunoresearch Laboratories) for 30 min, washed in PBS, and mounted in Vectashield containing DAPI (Vector Laboratories). For detection of neutrophils, cryosections were fixed in 4% paraformaldehyde in PBS for 10 min. Sections were then permeabilized and blocked in 0.2% Triton containing 1% donkey serum for 30 min and stained with a rabbit antibody against myeloperoxidase (Abcam) followed by secondary antibodies and washing steps as described above.
BALF cytokine analysis
A multiplex bead assay based on the Luminex technology was used to measure cytokine/chemokine levels in BALF samples that were collected from mice as described earlier. A 25-panel multianalyte cytokine/chemokine kit (Millipore) was used according to the manufacturer's instructions. Briefly, 25 μl of samples were incubated overnight at 4°C with capture beads against G-CSF, GM-CSF, IFN-γ, TNF-α, RANTES, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IP-10, KC, MCP-1, MIP-1α, MIP-1β, and MIP-2 on a rocking platform. The following day, beads were washed using a hand-held magnetic block (Millipore) and stained with biotin antibodies at room temperature for 1 hr. The beads were further stained for 30 minutes using streptavidin detection antibodies before a series of final washes. Beads were resuspended in sheath fluid and samples were read on a Luminex 200 instrument (Luminex), and the levels of each cytokine were determined by regressing against a seven-point standard curve (Bioplex Manager, Biorad).
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2007. The Student's t-test was used to determine significance of differences between two groups.
Results
To evaluate the effects of a chronic bacterial infection on gene transfer in the airway, C57BL/6 mice were i.n. challenged with approximately 105 CFU of Bb RB50 or sterile PBS. Mice were tested for evidence of infection through day 21 postinfection, and in all RB50-infected mice, bacteria were detected at significant levels (104–107 CFU) in both the nasal cavity and lung throughout the duration of the study (Fig. 2A). All samples from mice receiving only sterile PBS showed no evidence of RB50 at all time points tested (data not shown). On day 2 or day 14 of infection, mice were given an intranasal dose of AAV9.ffluc. One week after AAV administration, mice were subjected to live whole-animal luminescent imaging. At both time points, the luminescent signal in the nasal cavity of the infected mice was significantly lower (p<0.01, Student's t-test) than that observed in the nasal cavity of the uninfected mice (Fig. 2B and C). It is worthwhile to note that the loss in expression was greater (eightfold; Fig. 2C) when AAV administration was delayed to day 14 of infection. The significant difference in ffluc expression in the nasal cavity between uninfected mice and infected mice receiving AAV on day 14 was sustained for 50 days after AAV delivery (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum).
FIG. 2.
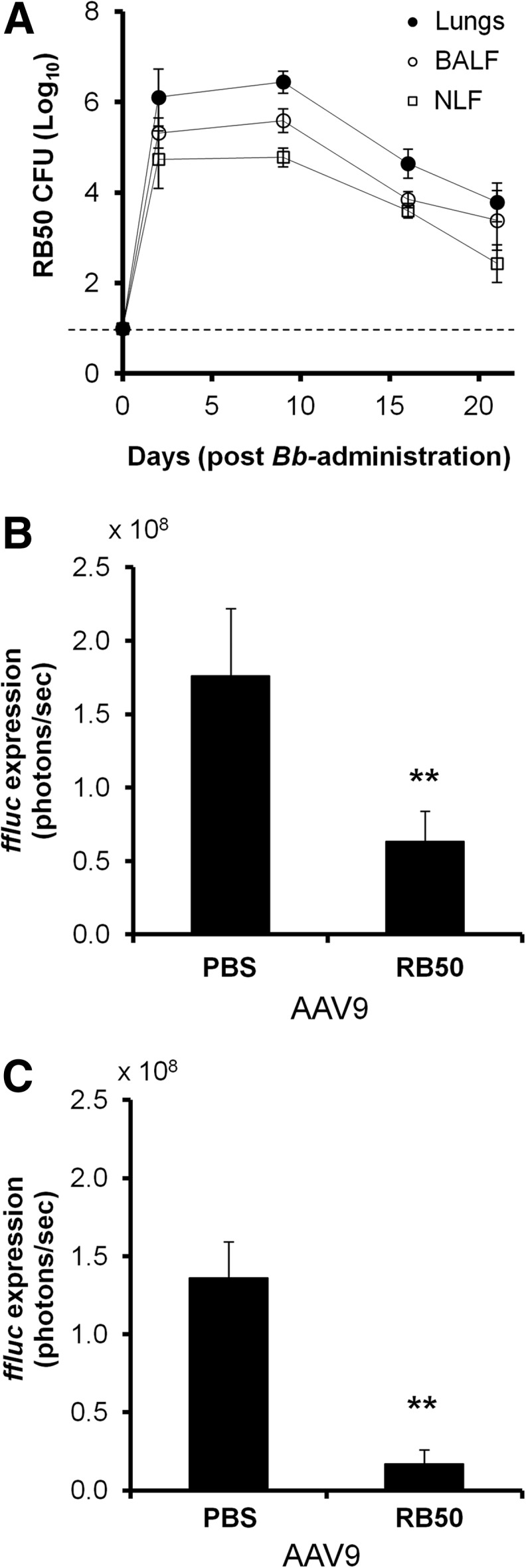
Effects of a sustained Bb RB50 airway infection on AAV9-mediated transduction in C57BL/6 mice. (A) RB50 colonization of C57BL/6 mouse airway. Approximately 105 CFU of bacteria were delivered i.n. in 30 μl. Bacterial colonization was analyzed through harvest of lungs, BALF, and NLF from 3 to 4 randomly selected mice at specified time points over the course of 21 days. The plots show means±standard deviations for n=4–11 mice tested at each time point in 4 different experiments. Samples taken from uninfected mice were clear of RB50 at all time points. Dashed line denotes detection limit. (B and C) AAV9-mediated transduction in wild-type C57BL/6 mice recently infected with RB50. Two or 14 days after receiving intranasal inoculations of either sterile PBS or ∼105 CFU of Bb, mice were i.n. dosed with AAV9.ffluc. Mice were assessed for gene expression 1 week later: (B) mice that received AAV on day 2 of infection and (C) mice that received AAV on day 14 of infection. Plots show quantification of luminescence from mouse nasal cavity. Results are presented as means, and error bars represent the standard deviation for the six mice imaged at each time point. Significantly lower signal was observed from the nasal cavities of infected mice (**p<0.01, Student's t-test, n=6). Bb, Bordetella bronchiseptica.
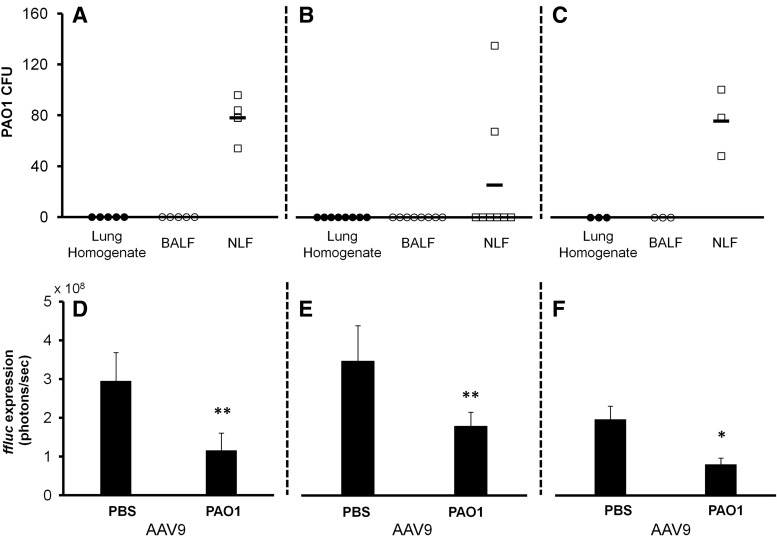
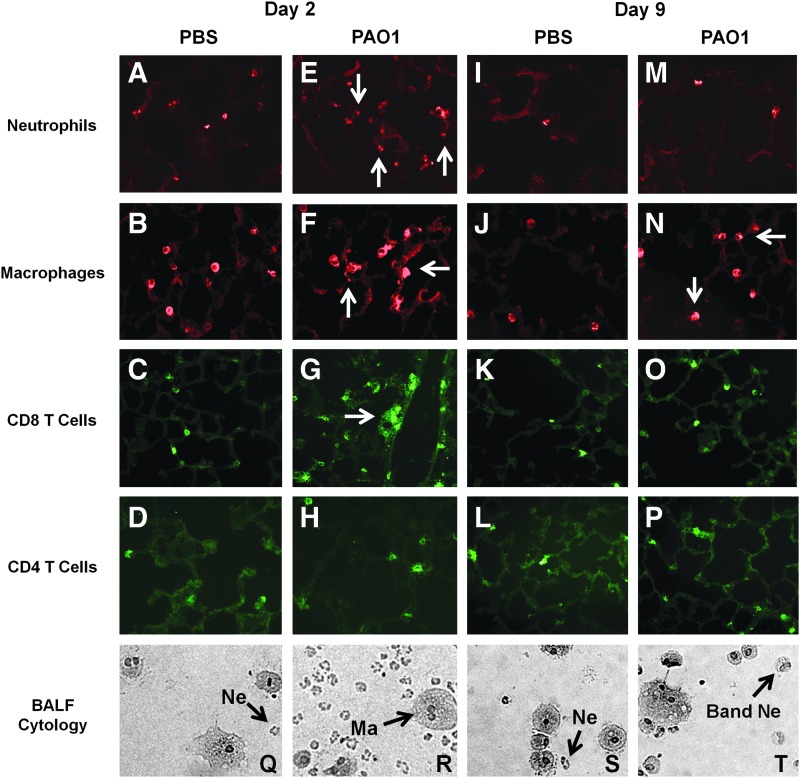
To determine whether a transient acute infection would have a similar effect, P. aeruginosa PAO1 was used to infect SP-D KO mice. Mice were i.n. challenged with approximately 105 CFU of PAO1 and control mice given sterile PBS. On day 2 or day 9 postinfection, a representative sampling of mice (uninfected n=3, infected n=4) was taken to determine the status of the infection in the lungs and nose at the time of AAV administration (Fig. 3A and B). Samples from uninfected mice showed no evidence of PAO1 at both time points tested (data not shown). On day 2, PAO1 was only found in the nasal cavity at relatively low levels (∼80 CFU). Lung and BALF samples isolated from the lower respiratory tract showed no evidence of infection. By day 9, the majority of mice had cleared the bacteria from their airways. However, despite the limited presence of bacteria in the nasal cavity, ffluc expression was still significantly lower (p<0.01, Student's t-test) in the noses of infected mice for both cases (Fig. 3D and E). Consistent observations were seen at subsequent time points through to day 14 posttransduction (Supplementary Figs. S2–S3).
FIG. 3.
Effects of a transient Pseudomonas aeruginosa PAO1 airway infection on AAV9-mediated transduction in SP-D or SP-A KO mice. (A–C) PAO1 colonization of SP-D or SP-A KO mouse airway. Approximately 105 CFU of bacteria were delivered i.n. in 30 μl. Bacterial colonization was analyzed through harvest of lungs, BALF, and NLF from three or four randomly selected mice at specified time points: (A) SP-D KO mice at day 2 postinfection, (B) SP-D KO mice at day 9 postinfection, and (C) SP-A KO mice at day 2 after infection. Plots show means±standard deviations for n=5–7 mice tested at each time point in 3 different experiments for SP-D KO mice and n=3 mice from a single experiment for SP-A KO mice. Samples taken concurrently from uninfected mice were clear of PAO1 at all time points tested. (D–F) AAV9-mediated transduction in SP-D or SP-A KO mice recently infected with PAO1. Two or 9 days after being challenged with sterile PBS or approximately 105 CFU of P. aeruginosa PAO1, mice were i.n. dosed with AAV9.ffluc. One week later, mice were imaged for gene expression: (D) SP-D KO mice that received AAV on day 2 of infection, (E) SP-D KO mice that received AAV on day 9 of infection, and (F) SP-A KO mice that received AAV on day 2 of infection. Plots show quantification of luminescence from mouse nasal cavity. Results are presented as means, and error bars represent the standard deviation for the mice imaged at each time point. Significantly lower signal was observed from the nasal cavities of infected mice (**p<0.01, *p<0.05, Student's t-test, uninfected n=6, infected SP-D KO mice receiving AAV on day 2 n=11, infected mice SP-D KO receiving AAV on day 9 n=8, n=7 for both SP-A KO mice groups). KO, knockout; SP-A, surfactant protein A; SP-D, surfactant protein D.
To determine whether infection would have similar effects on gene expression in mice lacking SP-A, the SP-A KO mice were i.n. challenged with ∼105 CFU of PAO1 or sterile PBS. On day 2 postinfection, mice (uninfected n=3, infected n=4) were peeled to determine the status of infection in the SP-A nasal and lung airways at the time of AAV administration (Fig. 2C). As observed in the SP-D KO mice, bacteria were found only in the nasal cavity at relatively low levels (∼80 CFU) on day 2 postinfection, and samples from uninfected mice showed no evidence of PAO1 (data not shown). Additionally, similar trends on reporter gene expression were also observed; ffluc expression was significantly lower (p<0.05, Student's t-test) in the nasal cavity of infected mice (Fig. 2F). Consistent observations were seen at subsequent time points through day 21 posttransduction (Supplementary Fig. SF4).
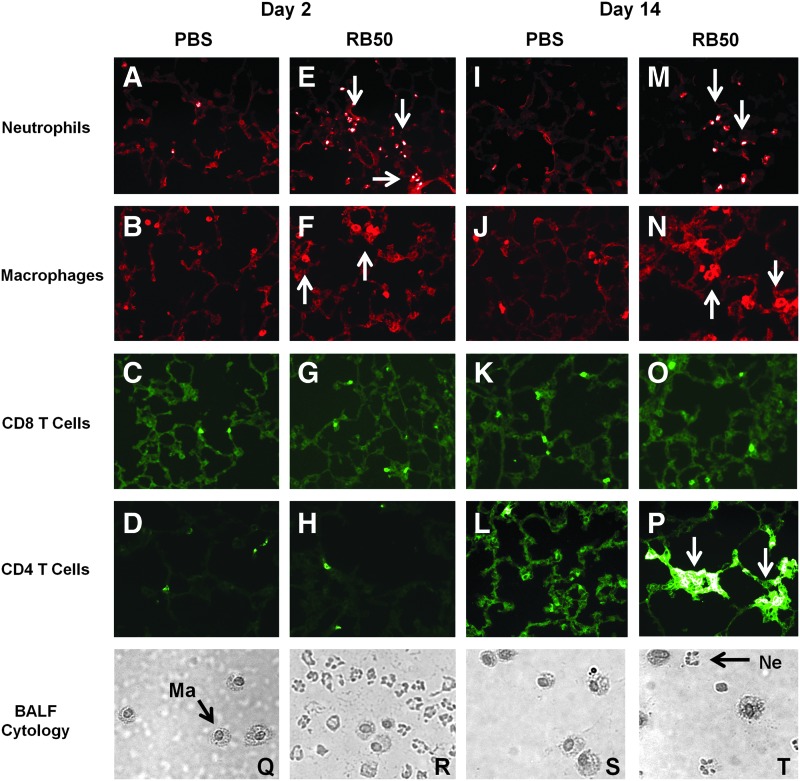
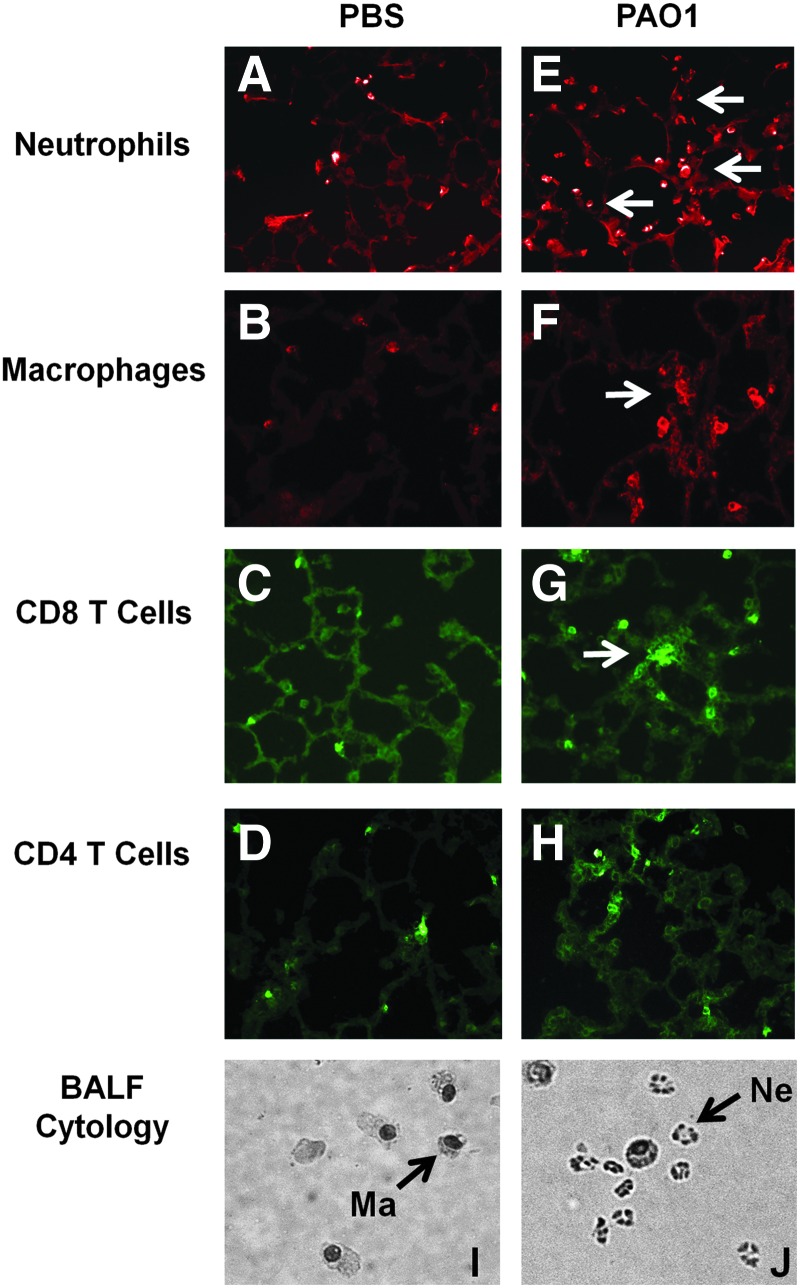
The immune response at the time of AAV administration was then characterized to provide insights into the observed mechanisms. Cytology on fresh BALF samples indicated that actively infected mice were characterized by substantial neutrophil infiltration on day 2 of infection (Figs. 4R, 5R, and 6J). Samples from uninfected mice only showed evidence of alveolar macrophages (Figs. 4Q, 5Q, and 6I). Interestingly, uninfected SP-D KO mice had a small number of neutrophils in their BALF samples in addition to macrophages that were characterized by a foamy and/or multinucleated appearance (Fig. 5Q). By day 9 post-PAO1 infection, many of the neutrophils had band, rather than segmented, nuclei (Fig. 5T), indicative of a shift toward immature precursors.
FIG. 4.
Characterization of immune response at time of AAV administration in Bb chronic infection model. (A–P) Lung histology of C57BL/6 mice transduced at specified time points during Bb airway infection. Lungs were harvested on the day of AAV administration and sectioned and stained with antibodies against antigens for the specified cell type. Representative images are shown here: sections from (A–D) uninfected mice on day 2, (E–H) infected mice on day 2, (I–L) uninfected mice on day 14, and (M–P) infected mice on day 14. Notable neutrophil and macrophage infiltration was observed in samples from infected lungs. Day 9 showed an elevated CD4 T cell population as well. Arrows indicate evidence of elevated cell populations. Magnification,×20. (Q–T) Cytospin preparations of cells recovered from fresh BALF samples taken on the day of AAV administration: (Q and S) samples taken from uninfected mice on day 2 and day 14, respectively; (R and T) samples taken from infected mice on day 2 and day 14, respectively. Representative macrophages (Ma) and neutrophils (Ne) are indicated in the figure. Samples from uninfected mice showed only macrophages, while samples from infected mice showed a prominent neutrophil presence in addition to the macrophage cell population at both time points. Color images available online at www.liebertpub.com/hum
FIG. 5.
Characterization of immune response at the time of AAV administration in SP-D KO/PAO1 acute infection model. (A–P) Lung histology of SP-D KO mice transduced at specified time points during PAO1 airway infection. Lungs were harvested on the day of AAV administration and sectioned and stained with antibodies against antigens for the specified cell type. Representative images are shown here: sections from (A–D) uninfected mice on day 2, (E–H) infected mice on day 2, (I–L) uninfected mice on day 9, and (M–P) infected mice on day 9. Day 2 samples from uninfected mice displayed evidence of neutrophils, macrophages, and CD8 T cells. Infected samples at this time point showed slightly elevated levels of the same cell types, as indicated by the arrows. By day 9, only macrophages remain slightly elevated. Magnification,×20. (Q–T) Cytospin preparations of cells recovered from fresh BALF samples taken on day of AAV administration: (Q and S) samples taken from uninfected mice on day 2 and day 9, respectively; (R and T) samples taken from infected mice on day 2 and day 9, respectively. Representative macrophages (Ma), neutrophils (Ne), and band neutrophils (band Ne) are indicated in the figure. Day 2 samples from uninfected mice showed macrophages and a small neutrophilic presence. Infected samples at this same time point displayed a much larger neutrophils population. By day 9, both macrophages and neutrophils were still seen. However, neutrophils on day 9 had band (instead of segmented) nuclei, indicative a shift toward immature precursors. Color images available online at www.liebertpub.com/hum
FIG. 6.
Characterization of immune response at time of AAV administration in SP-A KO/PAO1 acute infection model. (A–H) Lung histology of SP-A KO mice transduced on day 2 during PAO1 airway infection. Lungs were harvested on the day of AAV administration and sectioned and stained with antibodies against antigens for the specified cell type. Representative images are shown here: sections from (A–D) uninfected mice and (E–H) infected mice. On day 2, elevated levels of neutrophils, macrophages, and CD8 T cells were evident in samples from infected lungs (see arrows). Magnification,×20. (I–J) Cytospin preparations of cells recovered from fresh BALF samples taken on day of AAV administration: (I) samples taken from uninfected mice and (J) samples taken from infected mice. Representative macrophages (Ma) and neutrophils (Ne) are indicated in the figure. Samples from uninfected mice showed only macrophages, while infected samples showed obvious neutrophil infiltration in addition to the macrophage cell population. Color images available online at www.liebertpub.com/hum
Histology on frozen lung sections showed similar trends (Figs. 4–6). Day 2 of infection was characterized by significant neutrophil and macrophage infiltration for the Bb chronic infection and the PAO1 acute infection in the SP-A KO model (Figs. 4 and 6E–H). Day 2 of infection was also characterized by an elevated CD8 T cell population in the SP-A KO/PAO1 model (Fig. 6G). Interestingly, in the SP-D KO/PAO1 acute infection model, neutrophils and macrophages were present in the lungs of uninfected mice (Fig. 5A and B). We also observed small numbers of CD8 T cells in the uninfected lung and these increased slightly with infection. By day 9 postinfection with PAO1, lung samples from the SP-D KO mice showed little to no differences between uninfected and infected groups for all cell types detected (Fig. 5I–P). Day 14 of Bb infection in C57BL/6 mice, on the other hand, was characterized by elevated neutrophil and macrophage populations (Fig. 4M and N). Additionally, day 14 samples from infected lungs also showed a larger presence of CD4 T cells but not CD8 T cells (Fig. 4O and P).
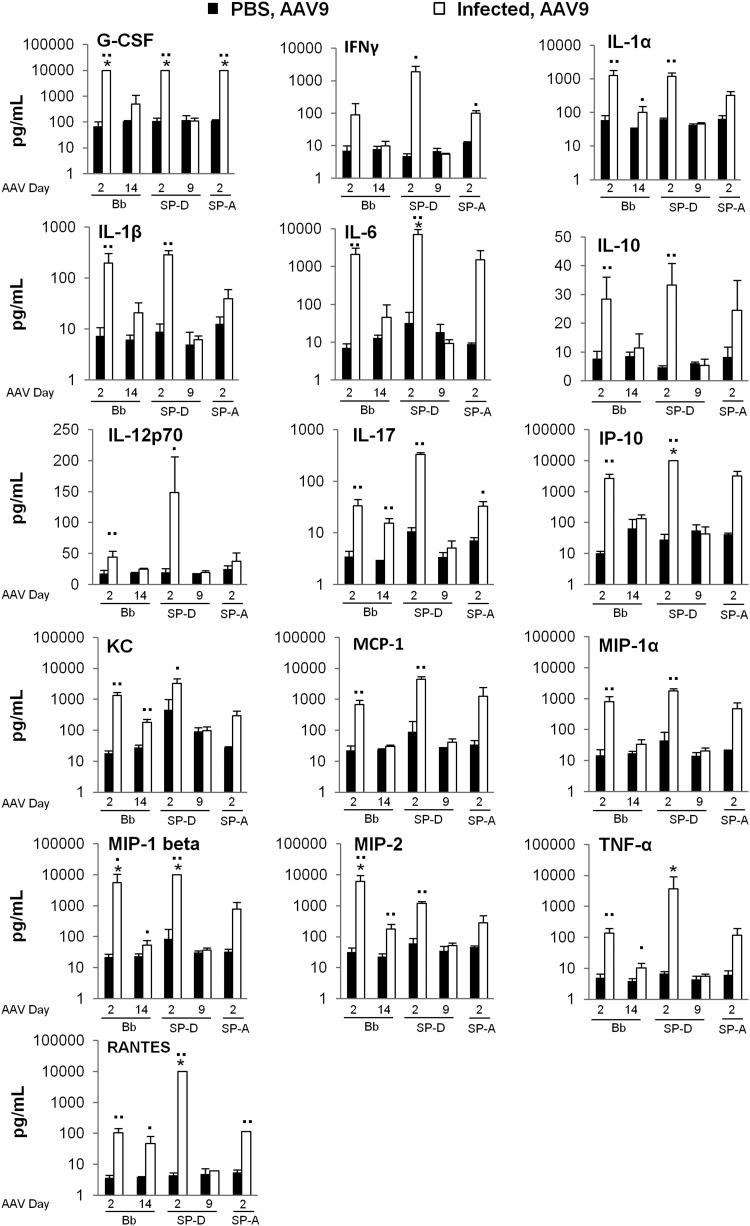
Analysis of cytokine/chemokine levels in BALF samples isolated from a representative sampling of mice (typically uninfected n=3, infected n=4) at the time of AAV administration demonstrated the most notable differences on day 2 of infection for all infection models (Fig. 7). Of the 25 analytes measured, 9 of them (GM-CSF, IL-2, IL-4, IL-5, IL-7, IL-9, IL-12p40, IL-13, and IL-15) showed no differences between uninfected and infected groups in any of infection models at any time points used in this study (data not shown). The remaining 16 (G-CSF, IFN-γ, IL-1α, IL-1β, IL-6, IL-10, IL-12p70, IL-17, IP-10, KC, MCP-1, MIP-1γ, MIP-1β, MIP-2, TNF-α, and RANTES) displayed substantial differences between uninfected and infected groups on day 2 postinfection in at least one of the infection models tested (Fig. 7). Determination of statistical significance for differences observed in the SP-A KO/PAO1 model was limited by the number of samples available for analysis (n=2 for each group). At the later time points tested, that is, at day 14 after Bb infection and at day 9 after PAO1 infection, many of the elevated analytes (i.e., G-CSF, IFN-γ, IL-1β, IL-6, IL-10, IL-12p70, IP-10, MCP-1, and MIP-1γ) returned to baseline concentrations observed in samples from uninfected mice. Those that did not return to baseline included IL-1α, IL-17, KC, MIP-1β, MIP-2, TNF-α, and RANTES in samples obtained from Bb-infected mice. In the SP-D KO/PAO1 model, levels of all cytokines and chemokines had returned to baseline by day 9 postinfection.
FIG. 7.
BALF cytokine/chemokine levels at time of AAV administration. Cytokine/chemokine levels were determined using a Milliplex 25-plex premixed magnetic mouse cytokine/chemokine array and Luminex bead reader according to the manufacturer's instructions. Single asterisk (*) indicates samples that exceeded the upper limit of detection of the assay, approximately 10,000 pg/ml. Results are presented as means, and error bars represent the standard deviation (▪▪p<0.01, ▪p<0.05, Student's t-test, uninfected n=2–5, infected n=3–7 for all groups except SP-A KO infected mice, where n=2 for both uninfected and infected mice).
Discussion
CF airway disease remains an ideal candidate for treatment by gene therapy, despite its limited success seen so far. Individuals with CF are characterized by chronic respiratory infections that are primarily responsible for the high morbidity and early mortality rates associated with the disease. However, limited research has been done to determine the effects of active airway infections on pulmonary gene transfer. The lack of research on this topic is not surprising as no convenient animal model exists to study such effects. Three different mouse models were established in this study to remedy this issue. The first involved creating a chronic infection in C57BL/6 mice with a natural murine pathogen, Bb RB50. The second and third models utilized PAO1 to establish an acute, but persistent, infection in SP-D and, separately, SP-A KO mice, respectively.
When an AAV vector was administered to the airways of infected mice on day 2 postinfection, transduction efficiency was similarly reduced in all mouse models, despite remarkable differences in the duration and intensity of all infection models. The approximate threefold reduction in gene expression was seen regardless of several additional factors, including the bacterium used, the strain of mouse used, or the bacterial load at the time of AAV administration. This suggests that immune response at the time of vector administration was the main cause for the observed reduction in gene transfer efficiency.
Characterization of these immune responses at day 2 of infection showed that the responses in each infection model were generally similar to each other. Analyses showed that day 2 inflammatory responses were dominated by macrophage and neutrophil infiltration, the latter of which is characteristic of the infected CF lung (Makam et al., 2009). However, in the SP-D KO/PAO1 acute infection model, elevated levels of macrophages and neutrophils were present even in uninfected lung samples, suggesting that their presence alone is insufficient to reduce gene transfer.
While immune responses at day 2 were generally similar between all infection models studied, one striking difference was observed between the chronic and transient acute infection models. In the SP-D and SP-A KO/PAO1 infection models, CD8 T cell activation/recruitment was observed in the infected lungs. In the lungs of Bb-infected mice, the numbers of CD8 T cells were not elevated compared with uninfected mice. Despite this apparent difference, we observed a similar level of reduction in gene expression in all three infection models, suggesting that CD8 T cells are not directly involved in the reduction of gene transfer.
When AAV administration was delayed to a later time point postinfection, different effects on AAV-mediated gene expression were seen between the Bb chronic infection and the SP-D KO/PAO1 acute infection models. In the Bb chronic infection model, postponement of AAV administration to day 14 postinfection resulted in a significant (8-fold, p<0.01, Student's t-test) reduction in gene expression, when compared with the expression seen in uninfected controls. In the SP-D KO/PAO1 acute infection model, however, delay of AAV administration to day 9 postinfection resulted in a ∼2-fold reduction. The notable differences observed in the immune response at these time points include (1) elevated levels of macrophages, segmented neutrophils, and CD4 T cells in Bb-infected mice but not in PAO1-infected mice and (2) elevated levels of IL-1α, IL-17, KC, MIP-1β, MIP-2, TNF-α, and RANTES in Bb-infected mice but not PAO1-infected mice. While PAO1-infected SP-D mice did display elevated levels of neutrophils in their BALF samples, many of these cells showed a band, rather than a segmented, nucleus, indicative of immature neutrophilic precursors. Taken together, these results indicate that activated, mature neutrophils and CD4 T cells may be responsible for causing significant reduction in gene expression observed in the Bb-infected mice. In particular, the presence of the CD4 T cells may be playing the more significant role, as that cell type was not present at the earlier AAV administration time point (day 2 postinfection), which reduced gene expression by only ∼3-fold.
Additionally, it is worthwhile to note that one of the elevated cytokines on day 14 of the chronic infection, IL-17, is produced by CD4 T cells and has been implicated in the stimulation of airway mucin gene expression (Chen et al., 2003). This could suggest the development of an enhanced mucus barrier by day 14 of the chronic infection. Such a phenomenon could explain the significant loss in gene transfer efficiency at this later time point for AAV administration. The enhanced mucus layer would prevent transduction by hindering normal transport of vector to the target cells.
Regardless, it is likely that in all our models, the inflammatory microenvironment and cytokine release profile following infection resulted in the breaking of immune tolerance to AAV particles and/or their gene products. In several studies, AAV vector administrations targeting liver have proven to be safe and rarely elicit an immune response, even when challenged with an adenovirus expressing a similar transgene. However, co-administration of TLR ligands, including CpG-containing oligodeoxynucleotides (ODNS), resulted in loss in liver transgene expression (Breous et al., 2011; Martino et al., 2009). Additionally, CpG-containing ODNs have also been proven to be potent adjuvants for adenovirus-mediated vaccinations against certain tumor antigens (Rieger and Kipps, 2003; Salucci et al., 2006). In these studies, adenovirus vectors expressing the targeted antigens are coadministered with CpG ODNs in order to trigger a sufficient immune response that establishes immunity against those antigens. With this in mind, it would be unsurprising if the bacterial presence in our infection models is serving as a form of adjuvant, triggering sufficient immunity to the AAV vector and/or to the ffluc product to result in a diminished expression or visualization thereof.
In each of our models, free bacteria were administered to the nares of healthy mice to facilitate a prolonged infection of the respiratory tract. The administration of free bacteria contrasts with the established practice of artificially embedding bacteria in agar beads before depositing them into the airway through a tracheal incision. The methods presented here are thus simpler and less invasive. Additionally, our methods do not limit the infection to the conducting airways, which is not the case with methods involving embedded beads. Because of their size, the beads create a mechanical block to the bronchi, resulting not only in limited bacterial access to the respiratory airways but also in increased morbidity, unwanted lung damage, and potentially even collapse or closure of the lung (Wu et al., 2000).
To the best of our knowledge, this the first study to examine the effects of an active, naturally occurring respiratory infection on AAV airway gene transfer. Our findings clearly show that the efficiency of AAV-mediated gene transfer in the airway is negatively impacted by the presence of an on-going or recently resolved bacterial infection. These results underscore the importance of considering such circumstances when developing potential genetic treatments for relevant diseases like CF and potentially other airway diseases such as asthma. The methods validated here provide simple and minimally invasive techniques that will allow this type of infected environment to be more easily accounted for.
Supplementary Material
Acknowledgments
We gratefully acknowledge Christine Draper, Deirdre McMenamin, Marco Crosariol, and Yanqing Zhu at the Gene Therapy Program for technical assistance with these studies; Robert Bucki for guidance on bacterial assays; Yasuhiko Irie for providing Bb strain RB50; and the Penn Vector Core for providing the AAV9 vector preparations.
The work was supported in part by NIH HL-66565 (S.L.D), the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1321851 (M.M.), and P30-DK-047757 (J.M.W.).
Any opinions, findings, and conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the funding agencies.
Author Disclosure Statement
No competing financial interests exist.
References
- Bell C.L., et al. (2011). The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. J. Clin. Invest. 121, 2427–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birault V., Solari R., Hanrahan J., and Thomas D.Y. (2013). Correctors of the basic trafficking defect of the mutant F508del-CFTR that causes cystic fibrosis. Curr. Opin. Chem. Biol. 17, 353–360 [DOI] [PubMed] [Google Scholar]
- Bobadilla J.L., Macek M., Fine J.P., and Farrell P.M. (2002). Cystic fibrosis: a worldwide analysis of CFTR mutations—correlation with incidence data and application to screening. Hum. Mutat. 19, 575–606 [DOI] [PubMed] [Google Scholar]
- Botas C., et al. (1998). Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc. Natl. Acad. Sci. USA 95, 11869–11874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous E., Somanathan S., Bell P., and Wilson J.M. (2011). Inflammation promotes the loss of adeno-associated virus-mediated transgene expression in mouse liver. Gastroenterology 141, 348–357, 357. e1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj S.S., et al. (2010). Pseudomonas aeruginosa alginate promotes Burkholderia cenocepacia persistence in cystic fibrosis transmembrane conductance regulator knockout mice. Infect. Immun. 78, 984–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., et al. (2003). Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 278, 17036–17043 [DOI] [PubMed] [Google Scholar]
- Cheng O.Z., and Palaniyar N. (2013). NET balancing: a problem in inflammatory lung diseases. Front. Immunol. 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation (2012). Cystic Fibrosis Foundation—Patient Registry Annual Data Report. Bethesda, MD [Google Scholar]
- Davis P.B., Drumm M., and Konstan M.W. (1996). Cystic fibrosis. Am. J. Respir. Crit. Care Med. 154, 1229–1256 [DOI] [PubMed] [Google Scholar]
- Ferrari S., Geddes D.M., and Alton E.W.F.W. (2002). Barriers to and new approaches for gene therapy and gene delivery in cystic fibrosis. Adv. Drug Deliv. Rev. 54, 1373–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni E., et al. (2006). Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 34, 704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J.R., and Deretic V. (1996). Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60, 539–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueirard P., et al. (2003). Bordetella bronchiseptica persists in the nasal cavities of mice and triggers early delivery of dendritic cells in the lymph nodes draining the lower and upper respiratory tract. Infect. Immun. 71, 4137–4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann N., et al. (2005). Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect. Immun. 73, 2504–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie Y., and Yuk M.H. (2007). In vivo colonization profile study of Bordetella bronchiseptica in the nasal cavity. FEMS Microbiol. Lett. 275, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.W.R., Matthews D.a., and Blair G.E. (2005). Novel molecular approaches to cystic fibrosis gene therapy. Biochem. J. 387, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makam M., et al. (2009). Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs. Proc. Natl. Acad. Sci. USA 106, 5779–5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino A.T., et al. (2009). Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS One 4, e6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R.B., et al. (2004). Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 125, 509–521 [DOI] [PubMed] [Google Scholar]
- Moss R.B., et al. (2005). Randomized, double-blind, placebo-controlled, dose-escalating study of aerosolized interferon gamma-1b in patients with mild to moderate cystic fibrosis lung disease. Pediatr. Pulmonol. 39, 209–218 [DOI] [PubMed] [Google Scholar]
- Moss R.B., et al. (2007). Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 18, 726–732 [DOI] [PubMed] [Google Scholar]
- Oakland M., Sinn P.L., and McCray P.B. (2012). Advances in cell and gene-based therapies for cystic fibrosis lung disease. Mol. Ther. 20, 1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo, A.a., et al. (2012). Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle A.D., et al. (1999). Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis underlying the relative ineffectiveness of the cellular inflammatory response in killing invading bacteria in. Am. J. Respir. Cell Mol. Biol. 20, 90–98 [DOI] [PubMed] [Google Scholar]
- Rieger R., and Kipps T.J. (2003). CpG oligodeoxynucleotides enhance the capacity of adenovirus-mediated CD154 gene transfer to generate effective B-cell lymphoma vaccines. Cancer Res. 63, 4128–4135 [PubMed] [Google Scholar]
- Salucci V., et al. (2006). CD8+ T-cell tolerance can be broken by an adenoviral vaccine while CD4+ T-cell tolerance is broken by additional co-administration of a Toll-like receptor ligand. Scand. J. Immunol. 63, 35–41 [DOI] [PubMed] [Google Scholar]
- Sinn P.L., Anthony R.M., and McCray P.B. (2011). Genetic therapies for cystic fibrosis lung disease. Hum. Mol. Genet. 20(R1),R79–R86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heeckeren A.M., and Schluchter M.D. (2002). Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim. 36, 291–312 [DOI] [PubMed] [Google Scholar]
- Wu H., et al. (2000). Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Microbiology 146, 2481–2493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.