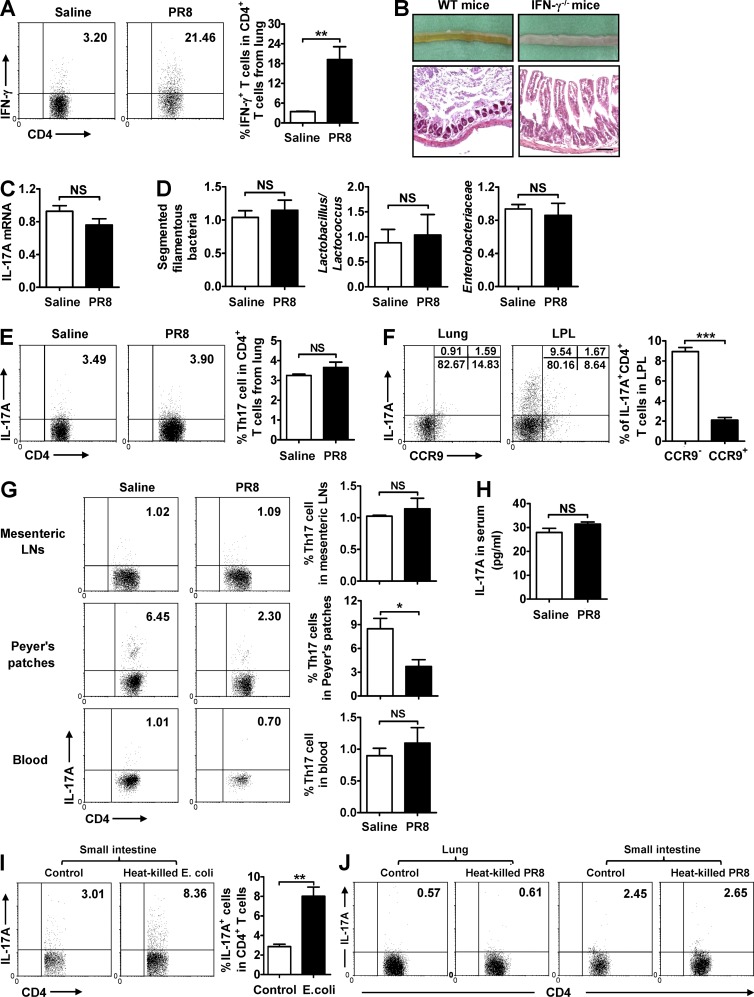
Figure 7.
Lung-derived CD4+ T cells influence microbiota and intestine injury by secreting IFN-γ. (A) IFN-γ expression in CD4+ T cells from lung was detected by flow cytometry 6 d after PR8 infection in WT mice. (B) The pathology of small intestine was assayed at day 7 after PR8 infection in WT and IFN-γ−/− mice, and tissue sections were stained with H&E. Bars, 100 µm. (C and D) IL-17A expression in the small intestine (C) and major bacterial groups in intestinal microbiota (D) were assayed at day 7 after PR8 infection in IFN-γ−/− mice. (E and F) IL-17A expression in CD4+ T cells from lung (E) and the percentages of CCR9− Th17 cells and CCR9+ Th17 cells in lung and LPL (F) were detected 6 d after PR8 infection in WT mice. (G) IL-17A expression in CD4+ T cells from mesenteric LNs, Peyer’s patches, and blood was detected by flow cytometry 6 d after PR8 infection in WT mice. (H) IL-17A level in serum was detected by ELISA 6 d after PR8 infection in WT mice. (I) LPL from WT mice at day 6 after PR8 infection was stimulated by heat-killed E. coli in vitro; 24 h later, the expression of IL-17A in CD4+ T cells was detected by flow cytometry. (J) Lung lymphocytes and LPL from WT mice at day 6 after PR8 infection were stimulated by heat-killed PR8 in vitro; 24 h later, the expression of IL-17A in CD4+ T cells was detected by flow cytometry. Data represent three independent experiments with three mice/group in A–H or three wells/treatment in I and J. Data are expressed as mean ± SEM by a Student’s t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS: not significant.