Abstract
The prevalence of Alzheimer’s disease (AD) is increasing rapidly, heightening the importance of finding effective preventive therapies for this devastating disease. Midlife vascular risk factors, including type 2 diabetes mellitus (T2DM), have been associated with increased risk of AD decades later and may serve as targets for AD prevention. Studies to date suggest that T2DM and hyperinsulinemia increase risk for AD, possibly through their effects on β-amyloid metabolism and cerebrovascular dysfunction - two early findings in preclinical AD pathology. This paper reviews the evidence supporting a relationship between T2DM, hyperinsulinemia, and diabetic dyslipidemia on the development of AD, discusses DM treatment trials and their preliminary results on cognitive function, and proposes some strategies for optimizing future AD prevention trial design.
Keywords: Alzheimer’s disease, diabetes mellitus, dyslipidemia, biological markers, cognition, prevention
INTRODUCTION
Alzheimer’s disease (AD) is a devastating illness that currently affects over 26 million people worldwide[1]. The prevalence of the disease is expected to increase fourfold over the next 40 years unless effective preventive therapies are discovered[1]. In addition, newer estimates of dementia prevalence worldwide suggest that previous calculations underestimated the true prevalence of the disease by approximately 10%[2]. The higher-than-expected rates of dementia may be due to not only the aging of the population, a key risk factor for AD, but also the increasing prevalence of specific dementia risk factors, including type 2 diabetes mellitus (T2DM)[3]. The lack of effective medical therapies to treat dementia coupled with the incipient projected dramatic increase in the number of persons with AD in the coming decades has put medical research in a crisis to urgently find effective treatment and prevention strategies.
While some well-established risk factors for AD cannot be altered, such as age and apolipoprotein E ε4 (APOE4) allele, midlife vascular risk factors that are modifiable have recently been identified as important risk factors for AD, making them attractive targets for AD preventive therapies[4–8]. In the presence of the current obesity epidemic and its subsequent contribution to climbing rates of T2DM and dyslipidemia, investigators are working to unravel the links between glucose, insulin, and lipid metabolism and their relationship to the underlying neurobiological changes noted in AD. This paper reviews the evidence supporting a relationship between T2DM, hyperinsulinemia, and diabetic dyslipidemia on the development of AD and proposes some strategies for optimizing investigations into whether treatment of these risk factors may delay or prevent the onset of AD in specific populations.
TYPE 2 DIABETES MELLITUS AS A RISK FACTOR FOR ALZHEIMER’S DISEASE
Epidemiologic Evidence – Relationship of Diabetes Mellitus with Incident Alzheimer’s Disease
While some longitudinal prospective studies support an association of T2DM and hyperinsulinemia with the development of AD, not all research confirms this relationship. In recent years, several systematic reviews have been published to summarize the data from longitudinal, population-based studies assessing the relationship between DM and incident dementia [9, 10]. A systematic review by Biessels and colleagues [9] published in 2006 included 11 prospective studies of varying quality that assessed late-life AD and DM. Of these 11 studies, seven showed a relationship between DM and incident AD, with relative risks, odds ratios, or hazard ratios ranging from 1.4 to 2.4 [6, 11–16]. Two additional prospective studies included in Biessels’ systematic review assessed the relationship of midlife diabetes with incident AD with one showing neutral results (RR 1.0, 95% CI 0.5–2.0) [17] and the other showing a strong relationship of midlife DM with AD (OR 4.4, p<0.01) [18].
A systematic review by Kopf and Frölich published in 2009 [10] summarized the findings of 11 prospective, population-based studies assessing the contribution of DM to risk of AD, including eight studies included in the systematic review by Biessels and colleagues. The studies reviewed in the analysis by Kopf and Frölich contained original data, ascertained DM at baseline, assessed AD as an outcome variable, and included some type of risk ratio in their statistical analyses [10]. Four out of eleven studies included in this systematic review showed an increased risk of AD in persons with DM with relative risks or hazard ratios ranging from 1.59 to 1.9 [6, 12–14]; the other seven studies showed non-significant results [11, 19–24]. Varying methodology may explain some of the discrepancies in findings between the studies as each study used different techniques to ascertain DM, including interview-based medical history, review of currently prescribed anti-diabetic medications, oral glucose tolerance tests, random blood glucose levels, and/or measurement of hemoglobin A1C [10]. Three out of four of the studies with significant findings had larger sample sizes of persons with DM (n > 600) and included oral glucose tolerance tests at baseline. Thus, the authors concluded that some studies with non-significant results may have been underpowered or may have missed DM in patients who had not yet been clinically diagnosed [10]. While in general the data analyzed in these two systematic reviews give credence to the hypothesis that T2DM may increase risk of incident AD, these reviews also highlight the importance of large sample sizes and standardized assessments of DM and other interrelated vascular risk factors in the design and analysis of future prospective studies.
Other prospective population-based studies have been published since Kopf and Frölich’s systematic review was completed. The Interdisciplinary Longitudinal Study on Adult Development and Aging (ILSE) evaluated a representative birth cohort of 381 subjects born between 1930 and 1932 in Germany. Compared to healthy subjects (n=159), persons with MCI (n=108) or AD (n=26) showed similar prevalence rates for T2DM (p=0.18) [25]. This study had very small numbers of persons with both AD and DM (n=6) or both MCI and DM (n=25), which may have reduced the power of the analysis and impacted study findings [25]. In a recent analysis of 1248 adults (ages ≥ 75 years) in the Kungsholmen project, investigators found that persons with DM (n=75) did not have increased risk of AD [26]. When they narrowed the analysis to the 11 adults with undiagnosed DM (defined as a random blood glucose ≥ 11.0 mmol/l [≥200 mg/dL] at baseline or HgbA1C≥ 6.4% at a follow-up examination) they found an association of increased risk of AD (HR 3.29, 95% CI 1.20–9.01) in those with undiagnosed DM. It is unclear if the discrepancy in findings between the total sample of persons with DM and the subgroup with undiagnosed DM represented true differences in risk related to diagnosis and treatment of DM or if the subgroup findings were driven by a few individuals within the small subgroup of participants. Larger studies are needed to confirm whether early diagnosis and treatment of T2DM impacts risk of progression to AD as such findings could impact screening and treatment recommendations for older adults at risk for T2DM and AD.
The timing of onset of T2DM may also influence risk for AD. In a case-control analysis using the Swedish Twin Registry (n=13,693 twin individuals), persons with DM (n=1,396) had an increased adjusted odds ratio for AD (n=56) (OR 1.69 [95% CI 1.16–2.36]) compared to those without DM [27]. The authors also found that onset of DM in midlife (n=16) (adjusted OR 2.25 [1.29–3.92]) conferred greater risk of AD compared to those with onset of DM at age ≥65 years (n=40) (adjusted OR 1.56 [1.05–2.32]) [27]. These findings are supported by other studies showing a relationship between midlife vascular risk factors and onset of AD decades later [4, 28–30]. The growing evidence that dementia risk begins in midlife or possibly even earlier may have significant public health implications.
T2DM may increase risk for progression from the “pre-dementia” condition of mild cognitive impairment (MCI) to AD. In a prospective study of 103 older adults with MCI recruited from primary care practices in London, DM was associated with an increased risk of progression to dementia (adjusted HR 2.9, 95% CI 1.1–7.3) [31]. Only 59% of the original participants were successfully followed for the 4-year duration of the study, however, leaving only16 people with both MCI and T2DM with six of those participants progressing to AD [31]. Larger studies are needed to confirm this finding that T2DM increases the risk of progression from MCI to AD.
Several other studies have investigated the effects of pre-diabetic conditions, such as “borderline DM” and hyperinsulinemia, on risk for AD and have found mixed results. In a Swedish cohort of 1,173 older adults, subjects with “borderline DM” (defined as no previous history of DM, no anti-diabetic medication, and random plasma glucose of 7.8 to 11.1 mmol/l [140 to 200 mg/dL]) had an increased risk of AD (adjusted HR 1.77, 95% CI 1.06–2.97) relative to the control population [32]. In an analysis of older adults from the Washington Heights-Inwood Columbia Aging Project population (n=683), hyperinsulinemia was associated with an increased risk of AD (HR 2.1, 95% CI 1.5–2.9) even in hyperinsulinemic subjects without DM (RR 2.3, 95% CI 1.5–3.6) [33]. In the Honolulu-Asia Aging Study of Japanese men (n=2,568), the risk of dementia (n=244, including AD and VaD) was increased at the two extremes of serum insulin levels, with persons with either very high or very low serum insulin levels having increased risk [34]. While this pattern persisted in the subgroup with AD, the results were not statistically significant. The authors proposed that these apparently discrepant results could be explained by high serum insulin levels contributing to increased pathologic changes associated with AD and low serum insulin levels serving as a marker of impending dementia [34]. In the population-based Uppsala Longitudinal Study of Adult Men (n=2322), participants had an intravenous glucose tolerance test measured at midlife and those men with impaired insulin secretion had a higher cumulative risk of AD (HR 1.30, 95% CI 1.06–1.56) [35]. These studies and other biological evidence support that peripheral hyperinsulinemia may contribute to AD onset. Larger prospective studies of pre-clinical middle-aged adults at risk for both DM and AD are needed to clarify the true risk of hyperinsulinemia and glucose intolerance on AD risk. Understanding the impact of borderline or undiagnosed T2DM on risk for AD and whether these risk factors begin to affect dementia risk in midlife may have important implications on the timing and widespread use of diabetic screening measures for AD prevention.
Epidemiologic Evidence – Relationship of Diabetes Mellitus with Cognitive Decline
Other studies have investigated the impact of DM on rates of cognitive decline in those both with AD and in those at risk for the disease. The prospective multicenter Réseau de la maladie d’Alzheimer – France (REAL.FR) study evaluated 608 community-dwelling persons with probable AD and MMSE score between 10 and 26 and followed them for a mean of 26 months [36]. Surprisingly, in the 63 patients (10.4%) with DM at baseline, cognitive decline was slower compared to those without DM (0.38, p=0.01). While the models used in this study adjusted for potential confounding factors such as hypertension, coronary heart disease, hypercholesterolemia, and atrial fibrillation, the authors note that they did not adjust for use of medications that are more commonly prescribed in diabetics (such as angiotensin converting enzyme [ACE] inhibitors, statins, and aspirin) which may potentially have positive cognitive effects [36–38]. In addition, use of self-report to assess vascular risk factors, including T2DM, hypertension, and hypercholesterolemia, may have influenced the study outcome. Other studies have found conflicting results, showing that T2DM is associated with changes in learning and memory, mental flexibility, and processing speed [39, 40] and that the rate of cognitive decline is accelerated in older adults with T2DM [41]. Further studies are necessary to clarify how T2DM affects AD progression as these findings may impact how aggressively DM is managed in persons with established AD.
Investigations support that pre-diabetic conditions, such as metabolic syndrome, may also increase risk for cognitive decline. Metabolic syndrome is a cluster of metabolic derangements that includes obesity (central adiposity), insulin resistance, glucose intolerance, dyslipidemia, and hypertension. In a 5-year prospective study of 2632 older men and women, metabolic syndrome (n=1016) was associated with an increased risk of cognitive decline (multivariate adjusted RR, 1.20; 95% CI, 1.02–1.41) compared to those without metabolic syndrome [42]. Participants with both metabolic syndrome and high levels of inflammation had an even greater risk for cognitive decline (multivariate adjusted RR, 1.66; 95% CI, 1.19–2.32) [42]. In a multicenter trial of 4895 older women with osteoporosis, increasing numbers of components of the metabolic syndrome were associated with a 23% age-adjusted increase in the risk of developing cognitive impairment per unit increase in the number of components (OR, 1.23; 95% CI, 1.09–1.39) [43]. Thus, while metabolic syndrome may contribute to cognitive decline, further research is needed to clarify if this constellation of risk factors contribute to progression to AD neuropathology [44]. Furthermore, it is unclear whether all of the components of metabolic syndrome contribute equally to risk of cognitive decline.
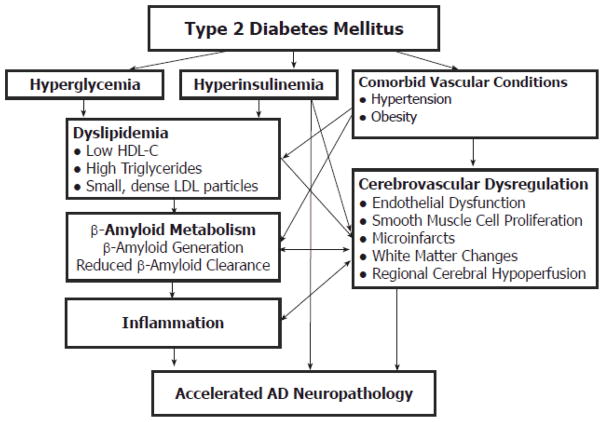
Given the strong association of T2DM with dyslipidemia, obesity, hypertension, physical inactivity, and inflammation, differentiating the impact of these risk factors on AD risk from those of DM may prove difficult (Figure 1). The Kungsholmen study showed interactive effects between DM and hypertension that affected the relative risk of AD [24]. In the Washington Heights-Inwood Columbia Aging Project population, persons with both hypertension and DM had an increased the risk of AD (HR, 3.3; 95% CI 1.9–5.9) compared to those without vascular risk factors [15]. Additional research will need to clarify the relative contributions of each vascular risk factor to AD risk in the presence of T2DM. As with cardiovascular risk assessment [45], the interactive effects of additional vascular risk factors with T2DM may potentiate the risk for AD. In addition, studies still need to clarify the potential impact of APOE4 allele carrier status, gender, and ethnicity on the relationship between T2DM and other vascular risk factors with AD risk [43, 46].
Figure 1.
Relationship of Diabetes Mellitus with Alzheimer’s Disease Pathology.
In summary, while methodological differences exist in prospective studies assessing the association of T2DM with incident AD and cognitive decline, evidence to date is strong enough to suggest that DM likely contributes to AD risk in subgroups of individuals. The association of midlife risk factors and undiagnosed DM with the onset of AD may lead to future changes in diabetic screening recommendations for persons at risk for DM and AD. While some prospective studies do support that hyperinsulinemia contributes to AD risk, more research is needed to better elucidate the various mechanisms through which T2DM contributes to development of AD.
Methodological Issues with Epidemiologic Studies
Many of the conflicting findings between various prospective epidemiologic studies may be due to methodological differences between studies, including variations in sample size, correction for increased mortality in diabetic subjects, definitions of DM, and adjustments for vascular confounding factors [10]. Given that T2DM is a risk factor for cardiovascular disease (CVD), stroke, and vascular dementia [12, 45], there may be some methodological issues regarding survival bias of non-diabetic patients with AD, especially in studies with long follow-up intervals [47]. In addition, each prospective study defined DM differently with some using random or fasting blood samples and others using self-reported diagnoses or assessment of anti-diabetic medication use. Since DM is underdiagnosed in older adults, studies without glucose measurements may have inappropriately assigned patients with T2DM to the non-diabetic group. In addition, while studying midlife vascular risk factors is attractive from a prevention perspective, studies that ascertain T2DM only at baseline in midlife may miss incident diabetes later in life and its impact on the development of AD. In addition to difficulties with standardization of the diagnostic criteria for T2DM in epidemiologic studies, there are also concerns about the standardized approach to diagnosing AD within large prospective studies. While many neuroepidemiologic studies now employ cognitive screening followed by a more detailed neurologic and neuropsychological assessment, some studies continue to use medical record review to diagnose AD.
In addition to methodological differences contributing to varying study outcomes, the tight relationship between T2DM with other important vascular risk factors make it difficult to assess the relative contribution of each of these factors to the development of AD. Not all studies have the ability to adjust for important confounding factors, including hypertension, dyslipidemia, obesity, depression, and other genetic factors [9]. Furthermore, epidemiologic studies may be confounded by medication use as persons with T2DM frequently use statins, aspirin, and ACE inhibitors – medications that may have beneficial cognitive effects [9, 37, 38, 48].
In order to provide the best data to clarify the relationship between DM and AD risk, future prospective studies will need to use standardized assessments of DM (including fasting blood samples), direct measures of related vascular risk factors (systolic and diastolic blood pressure, waist and hip measurements, fasting lipid panels, etc), and careful assessment of dose and duration of concomitant medication use. In addition, data quality will be improved with use of large sample sizes and frequent follow-up intervals to correctly adjust for survivor effect. Optimally, such studies will also include measures to clarify mechanisms of disease, such as blood insulin levels, cerebrospinal fluid (CSF) biomarkers for AD, and neuroimaging measures of perfusion and other pathologic changes.
MECHANISMS LINKING DIABETES MELLITUS, DYSLIPIDEMIA, AND ALZHEIMER’S DISEASE
Interrelationship Between β-Amyloid Metabolism and Cerebrovascular Dysfunction
AD neuropathology is characterized by the accumulation of extracellular amyloid plaques, composed chiefly of β-amyloid (Aβ), and intraneuronal neurofibrillary tangles, comprised of tau proteins. The aggregation and accumulation of cerebral Aβ may occur as a result of increased neuronal production, decreased activity of Aβ-degrading enzymes, or changes in the ability of Aβ to cross the blood-brain barrier [1]. Aβ accumulation subsequently leads to neuronal dysfunction and triggers the release of neurotoxic mediators [1]. In addition to the neurobiologic changes associated with Aβ, evidence supports that cerebrovascular dysregulation contributes to the pathogenesis of AD [7, 49]. Both Aβ deposition and cerebrovascular dysregulation are two early findings in preclinical AD pathology [50, 51] and work synergistically to accelerate neuronal degeneration [7]. (Figure 2) Physiological levels of soluble Aβ induce dysfunction in cerebral vessels of rats and in cultured endothelial cells [51, 52]. In animals, topical application of Aβ to the cerebral cortex leads to cerebrovascular dysregulation and systemic administration of Aβ reduces resting cerebral blood flow [53]. In a reciprocal manner, vascular risk factors and vascular dysfunction have also been shown to contribute to Aβ deposition [7, 54]. Thus, given the integrated relationship between Aβ generation and cerebrovascular dysfunction, therapeutic interventions that both reduce Aβ levels and improve cerebral blood flow may interrupt this cascade effect to delay the development of AD pathology.
Figure 2.

Interrelationship between β-Amyloid Metabolism and Cerebrovascular Dysregulation
Impact of Diabetes Mellitus on β-Amyloid Metabolism and Cerebrovascular Dysfunction
T2DM and dyslipidemia both affect Aβ generation and cerebrovascular dysfunction and, thus, are attractive targets for AD prevention strategies. T2DM is associated with a dyslipidemia characterized by hypertriglyceridemia, low HDL-cholesterol levels, and small, dense low-density lipoprotein (LDL) particles. T2DM, hyperinsulinemia, and dyslipidemia all contribute to abnormal Aβ metabolism [55, 56], endothelial dysfunction [57, 58], and increased arterial stiffness [59, 60] - central components of cerebrovascular dysfunction. Dysfunctional insulin signaling contributes to the pathogenesis of AD [61, 62], as insulin signaling regulates glucose metabolism in the brain and plays an important regulatory role in neuronal development, learning and memory [63, 64]. Insulin stimulates Aβ secretion and inhibits extracellular degradation of Aβ by competing for insulin-degrading enzyme (IDE) [65–67]. IDE is a key regulator of Aβ in neurons and glial cells. IDE knockout mice have hyperinsulinemia, glucose intolerance, and increased cerebral accumulation of Aβ [67]. In support of this relationship between high peripheral insulin levels and Aβ metabolism, insulin infusions (coupled with dextrose infusion to maintain euglycemia) in 16 older adults led to increases in cerebrospinal fluid (CSF) Aβ42 levels [68]. In a reciprocal manner, a transgenic mouse model demonstrated that the physiological role of β-amyloid precursor protein (AβPP) modulates glucose and insulin homeostasis of neurons [69].
In addition to insulin’s direct effect on Aβ metabolism, deregulation of glucose metabolism results in an accumulation of advanced glycation end products (AGEs) [61, 70–72], mitochondrial dysfunction [73, 74], and inflammation [75, 76] – factors that contribute to the development of both DM and AD. In an autopsy study, brains of persons with both AD and DM showed increased number of Aβ dense plaques and receptor for AGEs (RAGE)-positive and Tau-positive cells, higher AGEs levels and major microglial activation, compared to persons with AD who did not have DM [72].
In addition to its effects on Aβ secretion and degradation, T2DM also contributes to cerebrovascular dysfunction via microvascular ischemia and endothelial dysfunction, conditions which may contribute to chronic cerebral hypoperfusion [58, 77]. Aging and insulin-like growth factor-1 receptor (IGF-1R) signaling are both related to the development of cerebrovascular dysfunction, DM, and AD [78]. T2DM leads to thickening of the capillary membrane, microinfarcts, generalized atrophy, and white matter changes [79]. These changes in the microvascular circulation may lead to reduced regional cerebral blood flow that impairs cerebral protein synthesis, a key factor for learning and memory [7, 80, 81]. By impairing protein synthesis, chronically reduced resting cerebral blood flow may trigger neuronal dysfunction, and, thus, alter cognitive function. The relationship between cerebrovascular changes, DM, and AD is supported by autopsy findings from the community-based Adult Changes in Thought Study [82]. This study evaluated incident dementia cases that underwent autopsies (n = 259) and had information on DM status (n = 196) to assess the impact of DM on neuropathological changes. The investigators divided autopsy cases into four groups: those without DM or dementia; those with DM, but without dementia; those without DM, but with dementia; and those with both DM and dementia. In persons with dementia (n=71), the investigators observed two patterns of injury by their DM status: individuals without DM had a greater Aβ load, while patients with DM had more microvascular infarcts. In diabetic patients with dementia, the number of microvascular infarcts was greater in deep cerebral structures in patients whose DM was treated, whereas amyloid plaque load tended to be greater for untreated diabetic patients [82]. These findings suggest that patients with DM have neuropathological findings consistent not only with possible vascular dementia (VaD), but also those consistent with AD, especially in untreated diabetic patients. The apparent discrepancy in findings of studies showing increased [72] versus decreased Aβ load [82] in persons with both DM and AD suggests that further investigation is warranted.
To further assess such vascular changes in clinical trials, investigators are increasingly using dynamic measures of cerebral macrovascular and microvascular function, such as transcranial Doppler, arterial spin-labeling MRI and single photon emission compute tomography (SPECT) imaging [83–85]. Integration of PET in vivo amyloid imaging into such trials as well, will provide a unique opportunity to assess how DM, hyperinsulinemia, and their treatments affect both Aβ metabolism and cerebrovascular reactivity and their relationship to AD.
Impact of Diabetes Mellitus on Other Pathologic Mechanisms
Glucose transport may also be related to tau pathology – the other key pathologic finding in AD. In a pathology study, levels of major brain glucose transporters O-GlcNAcylation and phosphorylation of tau were assessed in postmortem brain tissue from frontal cortices of controls, persons with T2DM, individuals with AD, and adults with both AD and DM [63]. Findings from this study suggested that DM contributes to increased risk for AD by impairing brain glucose uptake/metabolism and down-regulating O-GlcNAcylation which facilitates abnormal hyperphosyphorylation of tau [63]. Thus, DM may contribute to the onset of AD by both Aβ and tau-related mechanisms.
Diabetic Dyslipidemia and Alzheimer’s Disease Risk
Numerous prospective studies support that hypercholesterolemia is associated with an increased risk for AD [4, 86–88], although much less is known about the impact of hypertriglyceridemia, low HDL-C, and small, dense LDL particles characteristic of the lipid disorders noted in persons with DM. While animal models suggest that elevated plasma triglyceride levels may precede amyloid deposition in AD mouse models [89], human studies relating elevated triglyerides to AD are still controversial. Triglyceride levels were shown to interact with APOE4 genotype to influence risk for AD in Nigerian Yoruba adults [46], but other studies have not supported this association. Some studies suggest that neither HDL-C nor triglyceride concentrations contribute to cognitive decline [90]. Data from the French Three-City (3C) cohort (n=7087) demonstrated that high triglyceride levels were the only component of metabolic syndrome that was significantly associated with the incidence of all-cause dementia (n=208 cases) (HR 1.45, 95% CI 1.05–2.00) and the subgroup of those with vascular dementia (n=40 cases) (HR 2.27, 95% CI 1.16–4.42); however, this relationship was not noted in the subgroup with AD (n=134 cases) (HR 0.90, 95% CI 0.57–1.43) [91]. Small dense LDL particles are more closely associated with VaD and atherogenic dyslipidemia than AD [92]. Given the difficulty in obtaining fasting lipid samples in large prospective longitudinal studies, many prospective studies have not collected triglyceride levels, but only non-fasting total cholesterol levels. Cross-sectional studies of the association of triglycerides or HDL-C with AD may be less accurate than midlife assessments of risk due to the changes noted in cholesterol levels prior to the onset of AD. Thus, further investigation into the impact of diabetic-related dyslipidemia on AD risk is needed to clarify whether elevated triglycerides and low HDL-C contribute to pathologic changes over and above those induced by insulin-related neuropathology.
TREATING TYPE 2 DIABETES MELLITUS TO DELAY ONSET AND PROGRESSION OF ALZHEIMER’S DISEASE
Understanding the strength of the association between T2DM and AD risk, the potential mechanisms explaining this relationship, and the contributing effects of related vascular risk factors to AD pathology will allow investigators to develop effective prevention trials targeting these mechanisms. Development of randomized, double-blind, controlled prevention trials evaluating lifestyle and/or pharmacologic interventions affecting insulin sensitization and glucose metabolism are critical to clarifying whether careful management of DM can prevent or delay the onset and progression of AD. Studies are also needed to evaluate whether treatment of other related vascular conditions associated with T2DM, such as dyslipidemia and hypertension, will provide added benefit in delaying AD onset in persons with DM.
Early clinical trial results suggest that some insulin sensitizing agents may favorable modify AD progression. In a placebo-controlled, double-blind, parallel-group pilot study, 30 subjects with mild AD or amnestic mild cognitive impairment were randomized to a 6-month course of rosiglitazone (n = 20) or placebo (n = 10) [93]. Primary endpoints were cognitive performance and plasma Aβ levels. Relative to the placebo group, subjects receiving rosiglitazone exhibited better delayed recall and selective attention. Plasma Aβ levels were unchanged from baseline for subjects receiving rosiglitazone, but declined for subjects receiving placebo, consistent with reports that plasma Aβ42 decreases with progression of AD [93]. In a 24-week randomized controlled Phase II dosing trial of 511 AD patients, long-acting rosiglitazone therapy (2mg, 4mg, or 8mg) was not associated with any significant improvement in performance on the Alzheimer’s Disease Assessment Scale - cognitive portion over placebo [94, 95]. However, in persons who did not carry the APOE4 allele, all three doses of therapy demonstrated an improvement over placebo; no such benefit was noted in APOE4 allele carriers. These results led to a Phase III pharmaceutical trial that is underway to evaluate the impact of extended-release rosiglitazone on cognitive function in persons with mild to moderate AD and without treated DM [95, 96]. In this ongoing study, careful attention is being given to the underlying APOE genotype in the design and analysis of results [95].
A recent randomized, open-controlled clinical trial in persons with mild AD and T2DM, randomized 42 patients to either the peroxisome proliferator-activated receptor gamma (PPARγ) agonist pioglitazone (n=21) or no treatment (n=21) for 6 months [97]. They evaluated the effects of pioglitazone vs. no treatment on cognition, regional cerebral blood flow, and plasma levels of Aβ40 and Aβ42 and found that the pioglitazone group improved on cognition and regional cerebral blood flow in the parietal lobe, while the control group showed no improvement. Plasma Aβ40/Aβ42 ratios increased in the control group, but not in the pioglitazone group. The results of this pilot study demonstrated that pioglitazone exhibited cognitive improvements [97]. Another study is investigating the safety and tolerability of pioglitazone in non-diabetic patients with AD and generating preliminary information on whether pioglitazone might delay the progression of the disease [98]. An NIH-sponsored trial in overweight adults with MCI is evaluating the impact of metformin vs. placebo on memory and brain imaging outcome measures with MRI and FDG-PET [99]. Integration of biomarkers assessing Aβ metabolism and neuroimaging will facilitate the understanding of whether antidiabetic medications work to delay AD progression primarily through Aβ- or vascular-related mechanisms.
While peripheral hyperinsulinemia may lead to AD pathology, some evidence shows that intranasal insulin treatment, which travels directly into the central nervous system, may facilitate cognition in patients with early AD or MCI. In a randomized controlled trial of 25 patients with early AD or MCI, twice daily intranasal insulin administration was associated greater performance in delayed verbal memory, attention, and functional status compared to placebo. Insulin treatment raised fasting plasma Aβ40/42 ratio (p = 0.0207), suggesting a potential effect on not only cognition, but also on Aβ metabolism [100].
While the above mentioned studies focused on persons with MCI and/or AD, future clinical trials focusing on the management of DM for primary prevention of AD are critical. A few large clinical trials have shown that lifestyle interventions and anti-diabetic medications, including metformin and rosiglitazone, prevent the development of T2DM in at-risk adults [101–103]. Two of these trials, the Finnish Diabetes Prevention Study and the Diabetes Prevention Program in the United States, have planned ancillary studies assessing the impact of these interventions on cognition [101, 102, 104]. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Memory in Diabetes Study (ACCORD-MIND) is underway to test whether there is a difference in the rate of cognitive decline and structural brain change in patients with DM treated with standard-care guidelines compared with those treated with intensive-care guidelines [105].
In an effort to better understand the impact of AD preventive therapies on not only cognitive outcomes, but also the neurobiologic changes associated with these therapies, many AD treatment and prevention trials are now integrating AD biomarkers into their study design. Use of CSF Aβ and tau levels [106], structural and functional neuroimaging [98, 99, 105], as well as sensitive cognitive test batteries [107, 108] focusing on memory and executive function will clarify how cognitive changes correlate with pathological changes. In addition, future trials will continue to need to measure and appropriately adjust for other vascular risk factors that could impact treatment outcomes, such as dyslipidemia, hypertension, and abdominal obesity.
CONCLUSIONS
Evidence to date supports that T2DM may increase risk of AD, even beginning in midlife, and that undiagnosed DM may put individuals at even greater risk of dementia. T2DM may increase AD risk through hyperinsulinemia and its contribution to Aβ and cerebrovascular dysregulation. While high serum total cholesterol levels have been associated with increased risk of AD, it is unclear if the high serum triglyceride and low HDL-C levels characteristic of diabetic patients contribute to AD risk beyond that conferred by insulin dysregulation. Clinical trials are underway to evaluate the impact of treatment and prevention of T2DM on risk for the development and progression of AD.
Future Directions
Given the increased cardiovascular risk in persons with T2DM, there are already strong indications for many patients with DM to be prescribed potential preventive therapies for AD, including statins, aspirin, and ACE inhibitors. This makes it difficult to conduct randomized clinical trials assessing the utility of these agents in delaying the onset of AD, given the ethical concerns about randomization to placebo. Thus, future clinical trials for AD prevention may focus on persons falling just below indications for these therapeutic agents, such as those with “borderline diabetes” or metabolic syndrome. In addition, these therapies will likely need to be assessed in both persons with established AD and MCI as well as those persons at risk for AD, but without any cognitive symptoms. Thus, future clinical trials will likely target pre-diabetic, pre-clinical persons at risk for AD.
Efforts to curb this growing epidemic of T2DM and its contribution to AD will require effective interdisciplinary research partnerships to study mechanisms of cell signaling to design new therapeutic agents, clinical trials to investigate the prevention and treatment of T2DM and pre-diabetic conditions, and effective community-based, culturally sensitive interventions to educate persons about the risks and management of T2DM. Hopefully, such research and community partnerships will lead to a reduction in the prevalence of both DM and AD.
Acknowledgments
Dr. Carlsson was supported in part through National Institute on Aging grant K23 AG026752.
This is Madison VA GRECC manuscript number #2010–03.
Footnotes
The author has no conflicts of interest to disclose.
References
- 1.Mucke L. Neuroscience: Alzheimer’s disease. Nature. 2009;461:895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 2.Prince M, Jackson J. [Accessed December 31, 2009.];World Alzheimer Report. http://www.alz.co.uk/research/files/World%20Alzheimer%20Report.pdf.
- 3.Estimated county-level prevalence of diabetes and obesity - United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1259–1263. [PubMed] [Google Scholar]
- 4.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137:149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 6.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 8.de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 9.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 10.Kopf D, Frolich L. Risk of incident Alzheimer’s disease in diabetic patients: a systematic review of prospective trials. J Alzheimers Dis. 2009;16:677–685. doi: 10.3233/JAD-2009-1011. [DOI] [PubMed] [Google Scholar]
- 11.Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Nomiyama K, Kawano H, Ueda K, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 12.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 13.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 14.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 15.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brayne C, Gill C, Huppert FA, Barkley C, Gehlhaar E, Girling DM, O’Connor DW, Paykel ES. Vascular risks and incident dementia: results from a cohort study of the very old. Dement Geriatr Cogn Disord. 1998;9:175–180. doi: 10.1159/000017043. [DOI] [PubMed] [Google Scholar]
- 17.Curb JD, Rodriguez BL, Abbott RD, Petrovitch H, Ross GW, Masaki KH, Foley D, Blanchette PL, Harris T, Chen R, White LR. Longitudinal association of vascular and Alzheimer’s dementias, diabetes, and glucose tolerance. Neurology. 1999;52:971–975. doi: 10.1212/wnl.52.5.971. [DOI] [PubMed] [Google Scholar]
- 18.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc. 2003;51:410–414. doi: 10.1046/j.1532-5415.2003.51117.x. [DOI] [PubMed] [Google Scholar]
- 19.Akomolafe A, Beiser A, Meigs JB, Au R, Green RC, Farrer LA, Wolf PA, Seshadri S. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol. 2006;63:1551–1555. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 20.Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, Tschanz JT, Norton MC, Pieper CF, Munger RG, Breitner JC, Welsh-Bohmer KA. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 21.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 22.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer’s disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 23.Tyas SL, Manfreda J, Strain LA, Montgomery PR. Risk factors for Alzheimer’s disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30:590–597. doi: 10.1093/ije/30.3.590. [DOI] [PubMed] [Google Scholar]
- 24.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 25.Toro P, Schonknecht P, Schroder J. Type II diabetes in mild cognitive impairment and Alzheimer’s disease: results from a prospective population-based study in Germany. J Alzheimers Dis. 2009;16:687–691. doi: 10.3233/JAD-2009-0981. [DOI] [PubMed] [Google Scholar]
- 26.Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer’s disease: a population-based cohort study. Diabetologia. 2009;52:1031–1039. doi: 10.1007/s00125-009-1323-x. [DOI] [PubMed] [Google Scholar]
- 27.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 29.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 30.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 31.Velayudhan L, Poppe M, Archer N, Proitsi P, Brown RG, Lovestone S. Risk of developing dementia in people with diabetes and mild cognitive impairment. Br J Psychiatry. 196:36–40. doi: 10.1192/bjp.bp.109.067942. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Qiu C, Winblad B, Fratiglioni L. The effect of borderline diabetes on the risk of dementia and Alzheimer’s disease. Diabetes. 2007;56:211–216. doi: 10.2337/db06-0879. [DOI] [PubMed] [Google Scholar]
- 33.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 34.Peila R, Rodriguez BL, White LR, Launer LJ. Fasting insulin and incident dementia in an elderly population of Japanese-American men. Neurology. 2004;63:228–233. doi: 10.1212/01.wnl.0000129989.28404.9b. [DOI] [PubMed] [Google Scholar]
- 35.Ronnemaa E, Zethelius B, Sundelof J, Sundstrom J, Degerman-Gunnarsson M, Berne C, Lannfelt L, Kilander L. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology. 2008;71:1065–1071. doi: 10.1212/01.wnl.0000310646.32212.3a. [DOI] [PubMed] [Google Scholar]
- 36.Sanz C, Andrieu S, Sinclair A, Hanaire H, Vellas B. Diabetes is associated with a slower rate of cognitive decline in Alzheimer disease. Neurology. 2009;73:1359–1366. doi: 10.1212/WNL.0b013e3181bd80e9. [DOI] [PubMed] [Google Scholar]
- 37.Carlsson CM, Gleason CE, Hess TM, Moreland KA, Blazel HM, Koscik RL, Schreiber NT, Johnson SC, Atwood CS, Puglielli L, Hermann BP, McBride PE, Stein JH, Sager MA, Asthana S. Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle-aged adults at risk for Alzheimer’s disease. J Alzheimers Dis. 2008;13:187–197. doi: 10.3233/jad-2008-13209. [DOI] [PubMed] [Google Scholar]
- 38.Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, Yasar S, Atkinson H, Robbins M, Psaty B, Goff DC., Jr Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009;169:1195–1202. doi: 10.1001/archinternmed.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- 40.Strachan MW, Deary IJ, Ewing FM, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- 41.Allen KV, Frier BM, Strachan MW. The relationship between type 2 diabetes and cognitive dysfunction: longitudinal studies and their methodological limitations. Eur J Pharmacol. 2004;490:169–175. doi: 10.1016/j.ejphar.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 42.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 43.Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66:324–328. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller M, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement Geriatr Cogn Disord. 2007;24:185–192. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part I: recent advances in prevention and noninvasive management. J Am Coll Cardiol. 2007;49:631–642. doi: 10.1016/j.jacc.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 46.Hall K, Murrell J, Ogunniyi A, Deeg M, Baiyewu O, Gao S, Gureje O, Dickens J, Evans R, Smith-Gamble V, Unverzagt FW, Shen J, Hendrie H. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66:223–227. doi: 10.1212/01.wnl.0000194507.39504.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson GS, Craft S. Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer’s disease. Eur J Pharmacol. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 48.Carlsson CM, Nondahl DM, Klein BE, McBride PE, Sager MA, Schubert CR, Klein R, Cruickshanks KJ. Increased atherogenic lipoproteins are associated with cognitive impairment: effects of statins and subclinical atherosclerosis. Alzheimer Dis Assoc Disord. 2009;23:11–17. doi: 10.1097/wad.0b013e3181850188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184–190. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 50.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 51.Price JM, Chi X, Hellermann G, Sutton ET. Physiological levels of beta-amyloid induce cerebral vessel dysfunction and reduce endothelial nitric oxide production. Neurol Res. 2001;23:506–512. doi: 10.1179/016164101101198758. [DOI] [PubMed] [Google Scholar]
- 52.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 53.Niwa K, Carlson GA, Iadecola C. Exogenous A beta1–40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20:1659–1668. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Sparks DL, Scheff SW, Hunsaker JC, 3rd, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 55.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Liu H, Yang J, Liu X, Lu S, Wen T, Xie L, Wang G. Increased amyloid beta-peptide (1–40) level in brain of streptozotocin-induced diabetic rats. Neuroscience. 2008;153:796–802. doi: 10.1016/j.neuroscience.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Drexler H, Zeiher AM. Endothelial function in human coronary arteries in vivo. Focus on hypercholesterolemia. Hypertension. 1991;18:II90–99. doi: 10.1161/01.hyp.18.4_suppl.ii90. [DOI] [PubMed] [Google Scholar]
- 58.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paris D, Town T, Humphrey J, Yokota K, Mullan M. Cholesterol modulates vascular reactivity to endothelin-1 by stimulating a pro-inflammatory pathway. Biochem Biophys Res Commun. 2000;274:553–558. doi: 10.1006/bbrc.2000.3174. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Huang Y, Li X, Xu M, Bi Y, Zhang Y, Gu W, Ning G. Association of arterial stiffness with HbA1c in 1,000 type 2 diabetic patients with or without hypertension. Endocrine. 2009;36:262–267. doi: 10.1007/s12020-009-9221-z. [DOI] [PubMed] [Google Scholar]
- 61.Jones A, Kulozik P, Ostertag A, Herzig S. Common pathological processes and transcriptional pathways in Alzheimer’s disease and type 2 diabetes. J Alzheimers Dis. 2009;16:787–808. doi: 10.3233/JAD-2009-0973. [DOI] [PubMed] [Google Scholar]
- 62.de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J Neurochem. 2009;111:242–249. doi: 10.1111/j.1471-4159.2009.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerozissis K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur J Pharmacol. 2008;585:38–49. doi: 10.1016/j.ejphar.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 65.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 66.Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 67.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, Schwartz MW, Plymate S, Craft S. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60:1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 69.Needham BE, Wlodek ME, Ciccotosto GD, Fam BC, Masters CL, Proietto J, Andrikopoulos S, Cappai R. Identification of the Alzheimer’s disease amyloid precursor protein (APP) and its homologue APLP2 as essential modulators of glucose and insulin homeostasis and growth. J Pathol. 2008;215:155–163. doi: 10.1002/path.2343. [DOI] [PubMed] [Google Scholar]
- 70.Sasaki N, Fukatsu R, Tsuzuki K, Hayashi Y, Yoshida T, Fujii N, Koike T, Wakayama I, Yanagihara R, Garruto R, Amano N, Makita Z. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am J Pathol. 1998;153:1149–1155. doi: 10.1016/S0002-9440(10)65659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamagishi S, Takeuchi M, Inagaki Y, Nakamura K, Imaizumi T. Role of advanced glycation end products (AGEs) and their receptor (RAGE) in the pathogenesis of diabetic microangiopathy. Int J Clin Pharmacol Res. 2003;23:129–134. [PubMed] [Google Scholar]
- 72.Valente T, Gella A, Fernandez-Busquets X, Unzeta M, Durany N. Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitus. Neurobiol Dis. 37:67–76. doi: 10.1016/j.nbd.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30:1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 74.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 75.Barzilaym JI, Freedland ES. Inflammation and its relationship to insulin resistance, type 2 diabetes mellitus, and endothelial dysfunction. Metab Syndr Relat Disord. 2003;1:55–67. doi: 10.1089/154041903321648252. [DOI] [PubMed] [Google Scholar]
- 76.Finch CE, Morgan TE. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr Alzheimer Res. 2007;4:185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- 77.Taguchi A. Vascular factors in diabetes and Alzheimer’s disease. J Alzheimers Dis. 2009;16:859–864. doi: 10.3233/JAD-2009-0975. [DOI] [PubMed] [Google Scholar]
- 78.Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 79.Johnson PC, Brendel K, Meezan E. Thickened cerebral cortical capillary basement membranes in diabetics. Arch Pathol Lab Med. 1982;106:214–217. [PubMed] [Google Scholar]
- 80.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 81.Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol. 2000;10:587–592. doi: 10.1016/s0959-4388(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 82.Sonnen JA, Larson EB, Brickell K, Crane PK, Woltjer R, Montine TJ, Craft S. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315–322. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Novak V, Last D, Alsop DC, Abduljalil AM, Hu K, Lepicovsky L, Cavallerano J, Lipsitz LA. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care. 2006;29:1529–1534. doi: 10.2337/dc06-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chertkow H, Black S. Imaging biomarkers and their role in dementia clinical trials. Can J Neurol Sci. 2007;34(Suppl 1):S77–83. doi: 10.1017/s031716710000562x. [DOI] [PubMed] [Google Scholar]
- 86.Evans RM, Emsley CL, Gao S, Sahota A, Hall KS, Farlow MR, Hendrie H. Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: a population-based study of African Americans. Neurology. 2000;54:240–242. doi: 10.1212/wnl.54.1.240. [DOI] [PubMed] [Google Scholar]
- 87.Jarvik GP, Wijsman EM, Kukull WA, Schellenberg GD, Yu C, Larson EB. Interactions of apolipoprotein E genotype, total cholesterol level, age, and sex in prediction of Alzheimer’s disease: a case-control study. Neurology. 1995;45:1092–1096. doi: 10.1212/wnl.45.6.1092. [DOI] [PubMed] [Google Scholar]
- 88.Solomon A, Kareholt I, Ngandu T, Wolozin B, Macdonald SW, Winblad B, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M. Serum total cholesterol, statins and cognition in non-demented elderly. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 89.Burgess BL, McIsaac SA, Naus KE, Chan JY, Tansley GH, Yang J, Miao F, Ross CJ, van Eck M, Hayden MR, van Nostrand W, St George-Hyslop P, Westaway D, Wellington CL. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant A beta in plasma. Neurobiol Dis. 2006;24:114–127. doi: 10.1016/j.nbd.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 90.Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raffaitin C, Gin H, Empana JP, Helmer C, Berr C, Tzourio C, Portet F, Dartigues JF, Alperovitch A, Barberger-Gateau P. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City Study. Diabetes Care. 2009;32:169–174. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watanabe T, Koba S, Kawamura M, Itokawa M, Idei T, Nakagawa Y, Iguchi T, Katagiri T. Small dense low-density lipoprotein and carotid atherosclerosis in relation to vascular dementia. Metabolism. 2004;53:476–482. doi: 10.1016/j.metabol.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 93.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 94.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 95.Roses AD. Commentary on “a roadmap for the prevention of dementia: the inaugural Leon Thal Symposium.” An impending prevention clinical trial for Alzheimer’s disease: roadmaps and realities. Alzheimers Dement. 2008;4:164–166. doi: 10.1016/j.jalz.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 96. [Accessed January 3, 2010.];Rosiglitazone (Extended Release Tablets) As Monotherapy In Subjects With Mild To Moderate Alzheimer’s Disease. http://clinicaltrials.gov/ct2/show/NCT00428090.
- 97.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 98. [Accessed January 3 2010];Pioglitazone in Alzheimer Disease. http://www.clinicaltrials.gov/ct2/show/NCT00982202?term=diabetes+AND+Alzheimer%27s+disease&rank=7.
- 99. [Accessed January 3 2010];Metformin in Amnestic Mild Cognitive Impairment (MCI) http://www.clinicaltrials.gov/ct2/show/NCT00620191?term=diabetes+AND+Alzheimer%27s+disease&rank=3.
- 100.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 101.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 102.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 104.Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer’s disease. J Alzheimers Dis. 2009;16:693–704. doi: 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williamson JD, Miller ME, Bryan RN, Lazar RM, Coker LH, Johnson J, Cukierman T, Horowitz KR, Murray A, Launer LJ. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol. 2007;99:112i–122i. doi: 10.1016/j.amjcard.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 106.Watson GS, Bernhardt T, Reger MA, Cholerton BA, Baker LD, Peskind ER, Asthana S, Plymate SR, Frolich L, Craft S. Insulin effects on CSF norepinephrine and cognition in Alzheimer’s disease. Neurobiol Aging. 2006;27:38–41. doi: 10.1016/j.neurobiolaging.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 107.Ferris SH, Aisen PS, Cummings J, Galasko D, Salmon DP, Schneider L, Sano M, Whitehouse PJ, Edland S, Thal LJ. ADCS Prevention Instrument Project: overview and initial results. Alzheimer Dis Assoc Disord. 2006;20:S109–123. doi: 10.1097/01.wad.0000213870.40300.21. [DOI] [PubMed] [Google Scholar]
- 108.Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64:1323–1329. doi: 10.1001/archneur.64.9.1323. [DOI] [PubMed] [Google Scholar]