Abstract
Background:
Secondary lymphedema is a common complication of cancer therapy, but options for treating lymphedema are essentially ineffective and limited. On the contrary, lymphangiogenic growth factors accelerate lymphangiogenesis and improve lymphedema.
Methods:
Rat tail models of lymphedema were assigned to groups that received either daily topical basic fibroblast growth factor (bFGF) or saline (control) groups. Tail volume was measured, and the function of the lymphatic system was evaluated as the fluorescence intensity of indocyanine green every 3 days. The mRNA levels of vascular endothelial growth factor (VEGF)-C and VEGF-D and the protein levels of VEGF-C were evaluated at postoperative days (PODs) 7, 14, and 28. The subcutaneous and deep areas and lymphatic vessel density were histologically determined at PODs 7, 14, and 28.
Results:
Tail volume was significantly larger in the control than in the bFGF group (P < 0.05). The intensity of indocyanine green fluorescence significantly decreased earlier in the bFGF group (P < 0.05). The mRNA and protein levels of VEGF-C were upregulated in the bFGF group at POD 14 (P < 0.01). Both subcutaneous and deep tissues gradually withered in both groups but more rapidly in the bFGF, than in the control group, reaching statistically significant differences in the subcutaneous and deeper areas at POD 14 (P < 0.05). Lymphatic vessel density was significantly higher in the bFGF than in the control group at POD 14 (P < 0.05).
Conclusions:
Topical bFGF induces lymphangiogenesis and improves lymphedema in the rat tail model.
The lymphatic system has important functions in maintaining tissue fluid homeostasis, and it plays an important role in the acquired immune response and lipid absorption.1–3 The lymphatic system is associated with several diseases that are becoming increasingly prevalent such as asthma, obesity, diabetes, cancer metastasis, and lymphedema.4–6 Despite the critical roles that this system performs, much less is known about the lymphatic vasculature than the blood vasculature.
Lymphedema is characterized by the excessive, regional interstitial accumulation of protein-rich fluid, tissue fibrosis, and susceptibility to infection. Secondary lymphedema develops after disruption or obstruction of the lymphatic system such as by filariasis or as a consequence of surgery and radiotherapy for cancer.7,8 About 30%–40% of patients who undergo lymph node dissection will develop lymphedema.9,10 The development of effective treatment options for lymphedema has been hindered by the fact that the etiology of this disorder remains unknown. Specifically, treatment for lymphedema still emphasizes the alleviation of symptoms and it is primarily based on manual lymphatic massage and tight-fitting garments designed to physically prevent fluid accumulation. These treatments are time consuming and permanent. On the contrary, advances in microsurgical techniques and liposuction offer hope for the treatment of this disorder, but results of these procedures have been mixed.11 Thus, despite substantial advances in surgical and conservation techniques, therapeutic options for the management of lymphedema are limited and mostly ineffective.8,12
The mechanisms of inflammatory lymphangiogenesis are currently being elucidated. Lymphatic fluid stasis activates the expression of endogenous danger signals such as high mobility group box 1 and heat shock protein 70.13 Endogenous danger signals activate Toll-like receptors 2, 4, and 9 on macrophages and contribute to sterile inflammation and lymphangiogenesis.14,15 The endogenous activation of Toll-like receptor can be either homeostatic or harmful, depending on the physiological milieu and context, and thus lymphangiogenesis facilitated in this manner can improve lymphedema.
Several attempts to treat lymphedema with lymphangiogenic growth factors have found accelerated lymphangiogenesis and improved lymphedema.12,16–24 Therefore, therapy with lymphangiogenic growth factor might be the most appropriate strategy for treating secondary lymphedema after lymphatic vessel damage due to surgery, infection, or radiation therapy. Lymphangiogenic growth factors include vascular endothelial growth factor (VEGF)-C, VEGF-D, VEGF-A, angiopoietin-1, hepatocyte growth factor, adrenomedullin, and basic fibroblast growth factor (bFGF).16,17,20–25
Accumulating evidence indicates that VEGF-C plays a critically important and crucial role in the regulation of lymphatic vessel growth,17,26–29 and transfer of a gene expressing VEGF-C promotes the formation of lymphatic vessels and ameliorates lymphedema in an animal model.17,30,31 Furthermore, bFGF directly21,32,33 and indirectly induces lymphangiogenesis through VEGF-C and VEGF-D mediation,33–35 and it has been widely applied to treat pressure, burns, and leg ulcers, since it was introduced into the Japanese market in 2001. Several articles have described the value of topical bFGF for treating these conditions.36–38
We postulated that topical bFGF induces the growth of lymphatic vessels and ameliorates lymphedema. We tested this hypothesis by assessing the effect of bFGF on lymphangiogenesis and secondary lymphedema in a rat tail model.
MATERIALS AND METHODS
Animal Models
Eight-week-old male Lewis rats (Charles River Laboratories Japan, Yokohama, Japan) weighing 220–270 g (n = 162) were cared for according to the principles and guidelines of the Japanese Ministry of the Environment. The Ethics Committee for Animal Experiments at Nagoya University approved the study protocol (24442, 25036).
Rat Model of Secondary Lymphedema
We surgically created secondary lymphedema in rat tails on day 0 as described.39,40 General anesthesia was induced and maintained by a mixture of oxygen and isoflurane. Briefly, a full-thickness circumferential skin excision was positioned 20 mm from the tail base to remove the superficial lymphatic vessels, and then deep lymphatic vessels were ligated and cut using a surgical microscope after a subcutaneous injection of 0.1% Evans blue dye. Both skin edges were cauterized with a radio knife for hemostasis and to delay wound closure.
After surgery, rats were sprayed with either saline (controls) or bFGF as described below.
Topical bFGF Application
Recombinant human bFGF in 100 μg/mL of reconstitution fluid (Fiblast Spray, Trafermin, Kaken Pharmaceutical Co. Ltd., Tokyo, Japan) was sprayed using attached equipment to ensure uniform application to the surgical site as described by the manufacturer. The optimal concentration of bFGF was determined based on nonclinical studies.41 The surgical sites were directly sprayed with bFGF every day from postoperative day (POD) 1 to POD 28, and then 0.1% GENTACIN (gentamicin sulfate) ointment (MSD K.K., Tokyo, Japan) was applied 30 seconds later to prevent infection.
Tail Volume Measurement
Tail volume was measured as volumetric water displacement every 3 days (n = 15 per group). We measured tail volume 3 times at each time point, and the means of the 3 measurements were statistically analyzed. Rates of change were calculated as tail volume at each POD divided by preoperative tail volume. We also measured the tail volumes of age-matched rats that did not undergo surgery (n = 6) to estimate tail growth.
Lymphatic System Function
Lymphatic function was evaluated by measuring the fluorescence intensity of indocyanine green (ICG; Daiichi Sankyo, Tokyo, Japan) as described.42 We modified the method to evaluate lymphatic function more precisely. Briefly, 0.1 mg of ICG was subcutaneously injected into the end of the tail on POD 1. Fluorescence intensity gradually decreases as the injected ICG drains from the tail into the body through lymphatic ducts. The drainage function of the lymphatic fluid was evaluated by measuring the average fluorescence intensity at the distal part of the surgical site every 3 days using a PDE System C9830 infrared camera (Hamamatsu Photonics K.K., Hamamatsu, Japan). We evaluated 8 cm of the tail distal from the surgical site. Rates of change were calculated as the average fluorescence intensity at each POD divided by the average fluorescence intensity at POD 1. The fluorescence intensity of an age-matched group that did not undergo surgery was also measured (n = 6).
Real-time Polymerase Chain Reaction for VEGF-C and VEGF-D
Total RNA was isolated from tissue specimens (without bones or tendons) harvested from surgical sites (n = 6 per group) on PODs 7, 14, and 28 using RNeasy Lipid Tissue Mini Kits (Qiagen, Valencia, Calif.), and cDNA was prepared using High Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Foster City, Calif.). Specific primers for VEGF-C and VEGF-D TaqMan probes (Applied Biosystems) were used. The cDNA was amplified by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute followed by holding at 50°C for 2 minutes and 95°C for 10 minutes, and then differences in mRNA expression between the 2 groups were determined by calculating ΔCTs (CT for each target minus CT for β-actin for each sample). Levels of mRNA in the normal group (nonoperative, 9 weeks of age) were also measured, and the average was represented as 1. Relative values for the bFGF and control groups were then calculated.
Enzyme-linked Immunosorbent Assay for VEGF-C
All tissues except bone and tendon harvested from surgical sites (n = 6 per group) at PODs 7, 14, and 28 were homogenized in Tris-buffered saline containing 1% Nonidet P-40, 10% glycerol, and protease inhibitors. Samples were adjusted to the same protein concentration. Concentrations of VEGF-C proteins in the media were determined using a rat VEGF-C enzyme-linked immunosorbent assay kit (PromoKine, Heidelberg, Germany) according to the manufacturer’s instructions.
Histological Examination
Hematoxylin/Eosin Staining
The rats were killed at 7, 14, and 28 days after surgery, and surgical sites were excised along with 10 mm on the distal side. Tissues were fixed in 4% paraformaldehyde, decalcified in ethylenediaminetetraacetic acid, embedded in paraffin, and sectioned at 5-μm intervals 3 mm distal to the surgical site. Samples were histochemically stained with hematoxylin/eosin using standard techniques. Subcutaneous and deep-seated areas were measured using BZ-Analyzer software (Keyence K.K., Osaka, Japan). The subcutaneous area was considered to extend from the dermal/epidermal junction to the deep muscles, and the deep-seated area was considered to be the tissue underneath the subcutaneous area. Macrophage infiltration was confirmed using immunofluorescence staining with 5 μg/mL of anti-CD68 antibody (Abcam, Cambridge, United Kingdom).
Immunofluorescence Staining
The animals were killed at 7, 14, and 28 days after surgery, and surgical sites including 10 mm on the distal side were excised. Tissues were snap-frozen in isopentane cooled with liquid nitrogen and then embedded in frozen Super Cryoembedding Medium (Leica Microsystems, Tokyo, Japan) and sectioned at 4-μm intervals just distal to the surgical site. Sections were postfixed in 4% paraformaldehyde. Nonspecific binding on all sections was blocked using 5% goat serum in 0.01 M phosphate-buffered saline containing 0.3% Triton X-100 for 1 hour at room temperature. The sections were incubated with 4 μg/mL of anti–lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) antibody (Acris Antibodies GmbH, Herford, Germany) overnight at 4°C followed by 1:1000-diluted A11012 goat anti-rabbit Alexa 594 fluorescent antibody (Life Technologies, Gaithersburg, Md.) for 1 hour. Sections stained with LYVE1 were scanned at low magnification (×60) to select areas containing the most lymphatics (hot spots). Five hot spots within each tail section were counted at ×400 magnification, and the lymphatic vessel density was calculated as the mean number of lymphatic vessels in hot spots per field. The observer who selected the hot spots and counted lymphatic vessels was blinded to the treatment allocation of the rats. We confirmed that LYVE1 distinguished lymphatic endothelium from blood capillary cells by immunofluorescence double staining with 4 μg/mL of anti-LYVE1, 100 μg/mL of anti-CD31 antibody (Millipore, Billerica, Mass.), and 1:1000-diluted anti-mouse Alexa 488 fluorescent antibody (A11029; Life Technologies).
Statistical Analyses
Data were statistically analyzed by an unpaired t test using SPSS version 20 (SPSS Inc., Chicago, Ill.). The results are expressed as means ± SD. Differences were considered statistically significant at P < 0.05.
RESULTS
Tail Volume Measurement
Figure 1 shows representative photographs of rat tails in the treatment groups, and Figure 2 shows volumetric changes in the control (blue) and bFGF (red) groups and the physiological increases in tail volume during the experimental period (green). Although the 2 groups did not conspicuously differ on POD 7, differences became more obvious after POD 14. The trajectory of the tail volume data in the control group was consistently higher than in the bFGF group at all time points except on PODs 1 and 16. The difference became more conspicuous if physiological tail growth during the experimental period was also taken into consideration.
Fig. 1.
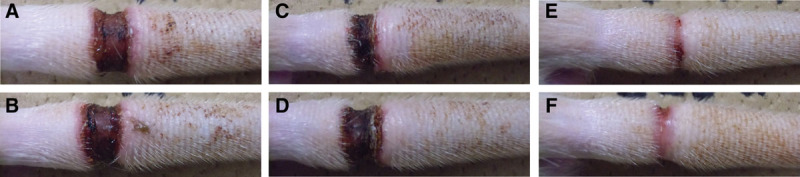
Representative photographs of rat tails in treatment groups. Swelling of the tail subsided sooner in the bFGF group than in the control group. A, bFGF-POD 7; B, Control-POD 7; C, bFGF-POD 14; D, Control-POD 14; E, bFGF-POD 28; F, Control-POD 28.
Fig. 2.
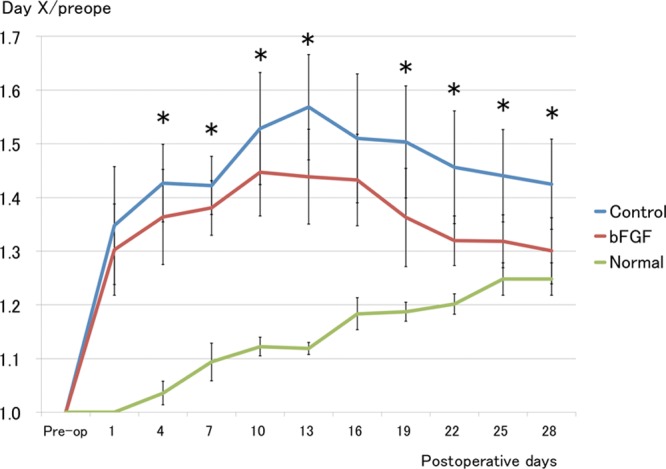
Tail volume measurements. Tail volume was smaller and decreased sooner in the bFGF group than in the control group. *P < 0.05 (t test). Control group and bFGF group: n = 15; normal group: n = 6.
Lymphatic System Function
The average fluorescence intensity rapidly decreased for up to 7 days and then gradually decreased in both groups. The trajectory of the bFGF group (red) remained lower throughout the experimental period. The average fluorescence intensity was significantly lower in the bFGF than in the control group (blue) on PODs 4 and 7 (P < 0.05), whereas lymphatic fluid smoothly drained in the normal group (green; Fig. 3).
Fig. 3.
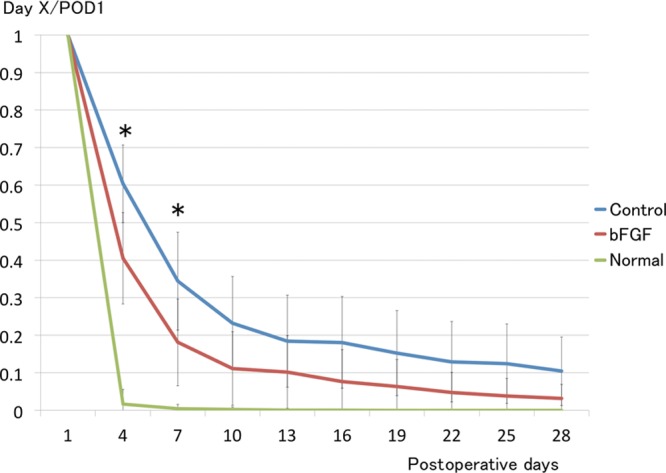
Fluorescent intensity of ICG. Intensity of ICG fluorescence decreased significantly sooner in the bFGF group during first week after surgery and remained lower throughout experimental period than in the control group. *P < 0.05 (t test). Each group: n = 6.
Real-time Polymerase Chain Reaction of VEGF-C and VEGF-D mRNA
More VEGF-C mRNAs were expressed in both groups on day 7, but the difference did not reach significance. Significantly more VEGF-C mRNAs were expressed in the bFGF than in the control group on POD 14 (P < 0.01; Fig. 4).
Fig. 4.
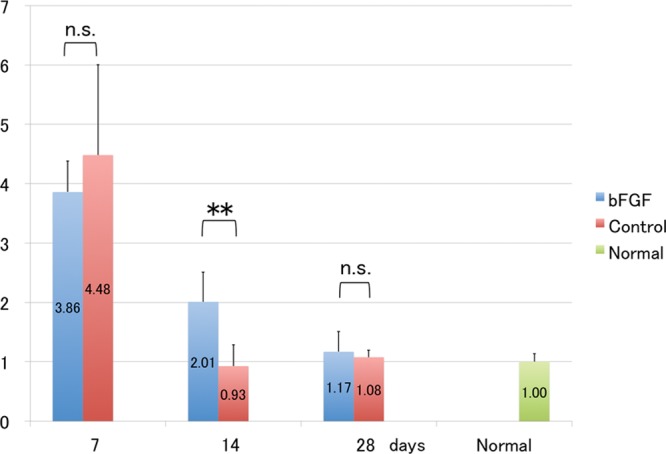
Messenger RNA levels of VEGF-C. Messenger RNA levels are enhanced in the bFGF group at POD 14. **P < 0.01 (t test). Each group: n = 6. n.s. indicates not significant.
Although VEGF-D mRNA expression was increased in both groups on day 7, differences between them were not significant on PODs 7, 14, and 28 (Fig. 5).
Fig. 5.
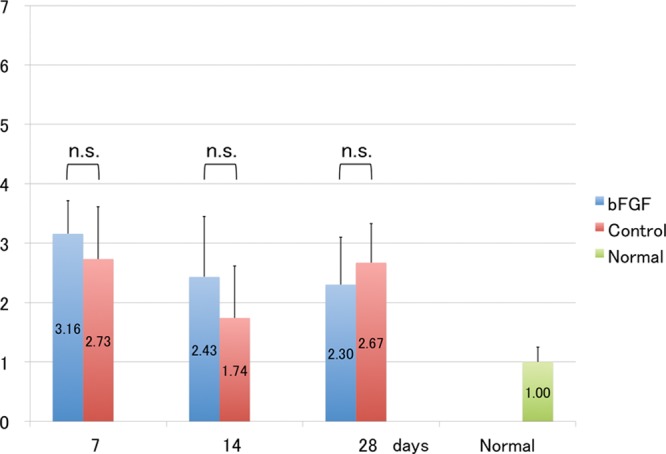
Messenger RNA levels of VEGF-D. Expression of VEGF-D mRNA is similar between bFGF and control groups. t test. Each group: n = 6. n.s. indicates not significant.
Enzyme-linked Immunosorbent Assays for VEGF-C
Protein levels in skin from homogenized surgical sites were determined using enzyme-linked immunosorbent assays. The protein level of VEGF-C was the highest in both groups on POD 14 and was significantly more increased in the bFGF than in the control group (P < 0.01; Fig. 6).
Fig. 6.
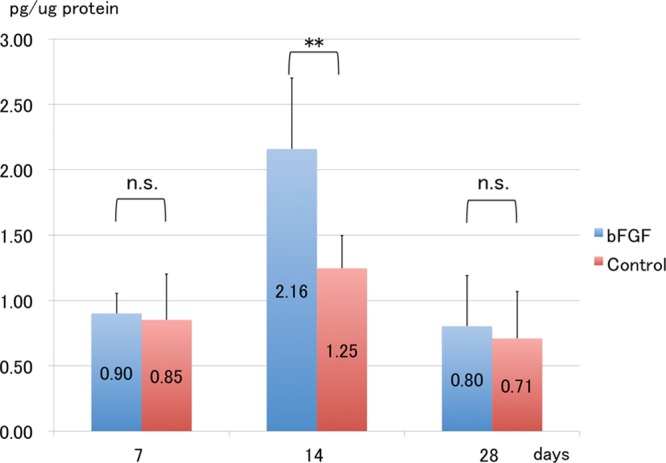
Protein levels of VEGF-C. Protein levels of VEGF-C are significantly increased in the bFGF group, compared with the control group on POD 14. **P < 0.01 (t test). Each group: n = 6. n.s. indicates not significant.
Histological Assessment
Subcutaneous and Deep-seated Areas
The subcutaneous and deep-seated tissues were responsible for most of the volumetric changes in the skin. Higher magnification showed modest infiltration by inflammatory cells (mostly macrophages) above the fasciae on PODs 7 and 14 and that inflammation subsided by day 28. In parallel with the inflammation, swelling peaked at day 14 and significantly decreased thereafter in both groups. Quantitative assessment revealed significantly less swelling in the subcutaneous and deep-seated tissues in the bFGF than in the control group on POD 14 (P < 0.05; Figs. 7, 8).
Fig. 7.
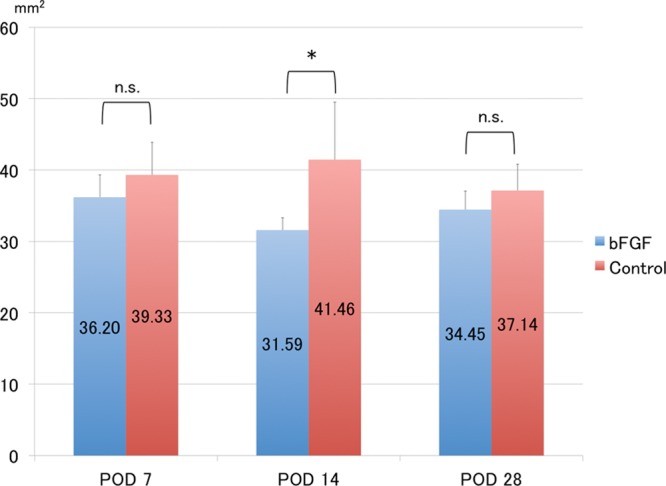
Measurement of subcutaneous areas. Subcutaneous area is significantly smaller in the bFGF group, than in the control group at POD 14. *P < 0.05 (t test). Each group: n = 6. n.s. indicates not significant.
Fig. 8.
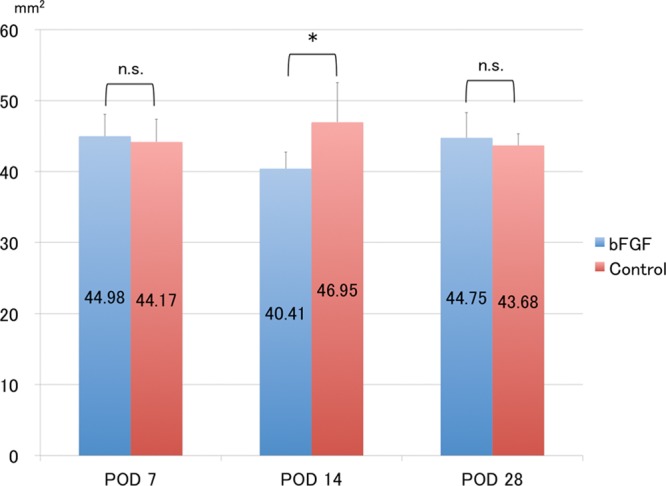
Measurement of deep-seated areas. Deep-seated area is significantly smaller in the bFGF group than in the control group at POD 14. *P < 0.05 (t test). Each group: n = 6. n.s. indicates not significant.
Lymphatic Vessel Density
Immunofluorescence analysis with LYVE1, a specific marker of lymphatic endothelial cells, demonstrated a serial increase in lymphatic vessel density after surgery (Fig. 9). In addition, quantitative assessment revealed a significant difference in lymphatic vessel density between the bFGF and control groups (Fig. 10). Furthermore, LYVE1-positive and CD31-positive cells were clearly distinguishable.
Fig. 9.
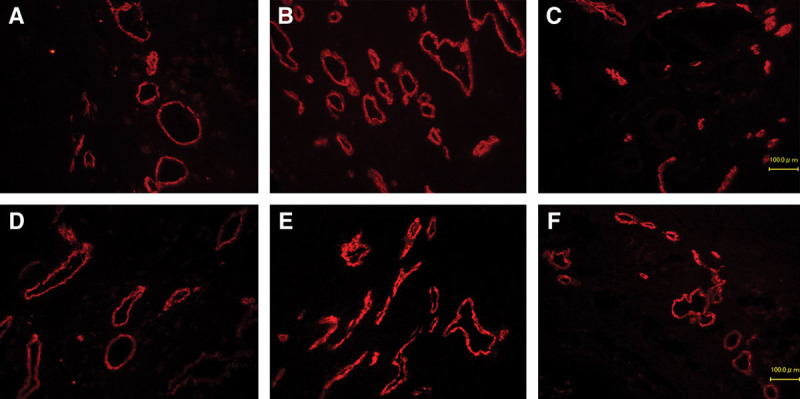
Immunohistochemical fluorescence staining for LYVE1. The numbers of visible lymphatic vessels (red) gradually increased over time. Lymphatic vessels are mainly located in subcutaneous tissue above fascia. A, bFGF-POD 7; B, bFGF-POD 14; C, bFGF-POD 28; D, Control-POD 7; E, Control-POD 14; F, Control-POD 28 (×100).
Fig. 10.
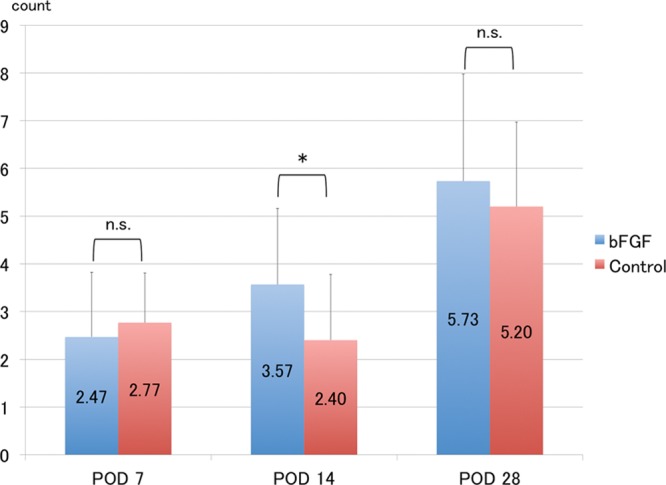
Measurement of lymphatic vessel density. Lymphatic vessel density gradually increased in both groups, but was significantly higher in the bFGF group than in the control group at POD 14. *P < 0.05 (t test). Each group: n = 6. Lymphatic vessel count/1 field, ×400. n.s. indicates not significant.
These findings suggest that sterile inflammation is temporarily induced in the subcutaneous tissue by acute lymphatic stagnation, which in turn stimulates lymphangiogenesis. Topical bFGF seemed to enhance lymphangiogenesis and thus enhance lymphatic drainage.
DISCUSSION
The pathogenesis of secondary lymphedema in humans is usually via the depletion of lymphatic vessels by radical surgery or radiotherapy for malignant tumors.7,8,12,43 Therefore, any treatment that can induce lymphatic vessel formation should be a promising option for this formidable condition. We showed here that topical bFGF induced lymphangiogenesis and significantly ameliorated secondary lymphedema through enhanced expression of VEGF-C at both the gene and protein levels in a rat model in vivo.
The VEGF family comprises a group of highly specific mitogens for endothelial cells. They induce angiogenesis and lymphangiogenesis through signal transduction involving tyrosine kinase receptors that lead to endothelial proliferation, migration, and vessel formation. The most studied of the group is VEGF-A, which binds to VEGF receptors (VEGFRs)-1 and -2 and induces angiogenesis, whereas VEGF-C and VEGF-D are potent lymphangiogenic factors that interact with VEGFR-329 that is expressed exclusively on lymphatic endothelial cells.16,44 Considerable experimental evidence has shown that these factors function in the development and maintenance of lymphatic systems.45 Mice deficient in VEGF-C have developmental loss of a functional lymphatic system,16 and the transgenic expression of soluble VEGFR-3 results in pronounced lymphedema.46 On the contrary, VEGF-C gene therapy ameliorates lymphedema through lymphangiogenesis in several animal models.17,30,31
The present study showed that topical bFGF facilitates lymphangiogenesis through VEGF-C upregulation. On POD 7, powerful upregulation of VEGF-C mRNA occurred naturally in both groups and was maintained with topical application of bFGF. In contrast to the control group, VEGF-C upregulation was observed in mRNA and protein levels in the bFGF group on POD 14. This promoted lymphangiogenesis and reduced tail volume. Upregulation subsided during wound healing, as recorded in both groups on POD 28. In addition, lymphedematous tail volumes were more attenuated in the bFGF, than in the control group, in parallel with a significant increase in the number of LYVE1-positive lymphatic vessels. Significantly less subcutaneous and deep-seated areas were found in the bFGF than in the control group on POD 14. These results indicate that topical bFGF improved secondary lymphedema through lymphangiogenesis facilitated by VEGF-C.
Circumferential measurements comprise the most popular clinical method of evaluating severity. However, secondary lymphedema occurs distal to lymphatic vessels or lymph node obstructions with a heterogeneous distribution.47 Therefore, volumetric changes provide more reliable parameters of severity. Here, we used the rat tail model of secondary lymphedema that allows precise volumetric analyses of the tail.40,42 We also measured tail volume 3 times at each time point, and the means of the 3 values were analyzed to further improve evaluation precision. The results showed that tail volume was consistently and significantly lower in the bFGF than in the control group at all time points except for PODs 1 and 16. The difference became more conspicuous when physiological growth of the tail was taken into account. This finding showed that topical bFGF improves lymphatic stagnation and enhances the restoration of lymphatic function.
bFGF is a potent mitogen for several cell types including blood vascular and blood capillary endothelial cells, and it induces an angiogenic response. Findings in vitro have indicated that bFGF should also directly21,32,33 and indirectly21,33,35 induce lymphangiogenesis. Others have shown a dose-dependent effect of bFGF on lymphatic endothelial cell proliferation, migration, and tube-like formation in vitro.21,48 The following has been proposed as possible induction mechanisms: a direct pathway via Phospatidylinositol 3-kinase/Akt49,50 and an indirect pathway associated with VEGF-C and VEGF-D that activates VEGFR-3. Accumulating evidence indicates that the signaling pathway of VEGF-C and its receptor, VEGFR-3, are critically important for the regulation of lymphatic vessel growth.17,26–29 Furthermore, bFGF can also increase tissue plasminogen activator activity and promote lymphatic endothelial cell motility.32 These findings indicated that bFGF induces lymphangiogenesis and improves secondary lymphedema in vivo.
We also evaluated lymphatic function by examining the rate at which injected ICG was removed from the distal part of the surgical site. Several recent studies have used near-infrared (NIR) fluorescence imaging with ICG to visualize lymphatic channels in vivo.51–54 Lymphography with ICG includes NIR imaging within the range of 700–900 nm, which enables deeper tissue penetration than visible light. ICG is the hallmark of NIR lymphatic imaging because it is approved by the Food and Drug Administration for use in humans. We measured ICG fluorescence intensity using an infrared camera and confirmed a statistically significant decrease in the fluorescence intensity of the bFGF, compared with the control group throughout the first postoperative week. Although the difference was not statistically significant at later time points, the ICG fluorescence intensity of the control group remained higher throughout the experiment period. These results indicate that topical bFGF is more effective during the early phase of lymphatic stagnation, although the effect seems to persist for at least 1 month. Therefore, we believe that bFGF treatment for secondary lymphedema should be implemented as soon as possible after lymphatic damage. On the contrary, bFGF and VEGF-C might enhance tumor growth and metastasis in tumor models because lymphatic circulation is a major route of the metastatic dissemination of cancer cells. In addition, the expression of the lymphangiogenic factors VEGF-C and VEGF-D correlates with the metastasis of many types of tumors in humans.55 However, although local injections of bFGF into tumor inoculation sites induce obvious tumor growth and metastasis, repeated bFGF injections at sites remote from the tumor do not.56 Thus, the clinical benefits and hazards of using local bFGF therapy to treat lymphedema caused by cancer therapy must be carefully considered. We envisage that bFGF therapy is indicated not only for secondary lymphedema, but also for postsurgical and posttraumatic swelling that interferes with rehabilitation and functional recovery. That is, the control of postsurgical and posttraumatic swelling might facilitate functional recovery and consequently improve outcomes.
The present study has some limitations. The rat tail model of secondary lymphedema might not replicate that in humans. The latter usually occurs insidiously, a significant delay in onset from lymphatic insult is quite frequent, and it seldom spontaneously recovers. In addition, less than one third of patients who have undergone radical lymphadenectomy for breast or uterine cancer develop secondary lymphedema. These features indicate that factors other than the mechanical obstruction of lymphatic flow are involved in the delayed development of chronic edema. In contrast, the rat tail model follows an acute course and spontaneously improves without treatment. Therefore, the beneficial effect of topical bFGF determined herein might not be generalizable to human secondary lymphedema. On the contrary, few would argue against the value of enhanced recovery from lymphedema during the acute phase after surgery in the context of preventing secondary lymphedema. Furthermore, although VEGF-C expression was enhanced at both the gene and protein levels, this does not necessarily guarantee that the VEGF-C pathway is solely responsible for the treatment effects determined in the present study. Therefore, further investigation is required to determine details of the mechanism using neutralizing antibodies against VEGF-C and bFGF.
CONCLUSIONS
Topical bFGF applied to surgical sites enhanced the recovery of lymphatic drainage and lymphangiogenesis after massive surgical lymphatic insult. Thus, bFGF can significantly ameliorate acute lymphatic edema although the long-term effects remain unknown.
ACKNOWLEDGMENTS
We thank Satoka Sugiura for technical assistance.
Footnotes
Disclosure: This work was supported by Japan Society for the Promotion of Science Grant Number 25462820. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Witte MH, Bernas MJ, Martin CP, et al. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55:122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- 2.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 3.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 4.Baluk P, Tammela T, Ator E, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 6.Ji RC. Characteristics of lymphatic endothelial cells in physiological and pathological conditions. Histol Histopathol. 2005;20:155–175. doi: 10.14670/HH-20.155. [DOI] [PubMed] [Google Scholar]
- 7.Browse NL. The diagnosis and management of primary lymphedema. J Vasc Surg. 1986;3:181–184. [PubMed] [Google Scholar]
- 8.de Almeida AB, Freedman DO. Epidemiology and immunopathology of bancroftian filariasis. Microbes Infect. 1999;1:1015–1022. doi: 10.1016/s1286-4579(99)80519-x. [DOI] [PubMed] [Google Scholar]
- 9.Rockson SG. Lymphedema. Curr Treat Options Cardiovasc Med. 2006;8:129–136. doi: 10.1007/s11936-006-0005-y. [DOI] [PubMed] [Google Scholar]
- 10.Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Cormier JN, Rourke L, Crosby M, et al. The surgical treatment of lymphedema: a systematic review of the contemporary literature (2004-2010). Ann Surg Oncol. 2012;19:642–651. doi: 10.1245/s10434-011-2017-4. [DOI] [PubMed] [Google Scholar]
- 12.Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vasc Med. 1998;3:145–156. doi: 10.1177/1358836X9800300209. [DOI] [PubMed] [Google Scholar]
- 13.Zampell JC, Yan A, Avraham T, et al. Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol. 2011;300:C1107–C1121. doi: 10.1152/ajpcell.00378.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zampell JC, Elhadad S, Avraham T, et al. Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am J Physiol Cell Physiol. 2012;302:C709–C719. doi: 10.1152/ajpcell.00284.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluwe J, Mencin A, Schwabe RF. Toll-like receptors, wound healing, and carcinogenesis. J Mol Med (Berl) 2009;87:125–138. doi: 10.1007/s00109-008-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 17.Yoon YS, Murayama T, Gravereaux E, et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest. 2003;111:717–725. doi: 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 19.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang LK, Garcia-Cardeña G, Farnebo F, et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 2004;101:11658–11663. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisada T, Oike Y, Yamada Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 23.Cao R, Björndahl MA, Gallego MI, et al. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 24.Jin D, Harada K, Ohnishi S, et al. Adrenomedullin induces lymphangiogenesis and ameliorates secondary lymphoedema. Cardiovasc Res. 2008;80:339–345. doi: 10.1093/cvr/cvn228. [DOI] [PubMed] [Google Scholar]
- 25.Haiko P, Makinen T, Keskitalo S, et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol. 2008;28:4843–4850. doi: 10.1128/MCB.02214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang D, Hu Y, Ling S. Expression of VEGF-C in rat cornea after alkali injury. J Huazhong Univ Sci Technolog Med Sci. 2004;24:483–485. doi: 10.1007/BF02831115. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Mäkinen T, Norrmén C, Petrova TV. Molecular mechanisms of lymphatic vascular development. Cell Mol Life Sci. 2007;64:1915–1929. doi: 10.1007/s00018-007-7040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohela M, Bry M, Tammela T, et al. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Szuba A, Skobe M, Karkkainen MJ, et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 2002;16:1985–1987. doi: 10.1096/fj.02-0401fje. [DOI] [PubMed] [Google Scholar]
- 31.Tammela T, Saaristo A, Holopainen T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 32.Liu NF, He QL. The regulatory effects of cytokines on lymphatic angiogenesis. Lymphology. 1997;30:3–12. [PubMed] [Google Scholar]
- 33.Pepper MS, Wasi S, Ferrara N, et al. In vitro angiogenic and proteolytic properties of bovine lymphatic endothelial cells. Exp Cell Res. 1994;210:298–305. doi: 10.1006/excr.1994.1042. [DOI] [PubMed] [Google Scholar]
- 34.Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol Med. 2002;8:483–489. doi: 10.1016/s1471-4914(02)02394-8. [DOI] [PubMed] [Google Scholar]
- 35.Kubo H, Cao R, Brakenhielm E, et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishibashi Y, Niimura M, Nishikawa T, et al. Investigation on clinical usefulness of recombinant human type bFGF (KCB-1) toward various pressure ulcer. J Clin Ther Med. 1993;9:2553–2570. [Google Scholar]
- 37.Ishibashi Y, Soeda S, Ohura T, et al. Evaluation of effect of recombinant human type bFGF (KCB-1) on pressure ulcer—The Phase III Clinical Trial Using Combination Drug of White Sugar/Povidone Iodine as Control Drug. J Clin Ther Med. 1996;12:2159–2182. [Google Scholar]
- 38.Soeda S, Ohura T, Shioya N, Tsukada S. Investigation on usefulness of bFGF (KCB-1) toward various pressure ulcer—The Early Phase II Clinical Trial Using 0.05% Solution. Jpn J Clin Exp Med. 1993;70:2660–2674. [Google Scholar]
- 39.Slavin SA, Van den Abbeele AD, Losken A, et al. Return of lymphatic function after flap transfer for acute lymphedema. Ann Surg. 1999;229:421–427. doi: 10.1097/00000658-199903000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito Y, Nakagami H, Morishita R, et al. Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis. Circulation. 2006;114:1177–1184. doi: 10.1161/CIRCULATIONAHA.105.602953. [DOI] [PubMed] [Google Scholar]
- 41.Okumura M, Okuda T, Nakamura T, et al. Acceleration of wound healing in diabetic mice by basic fibroblast growth factor. Biol Pharm Bull. 1996;19:530–535. doi: 10.1248/bpb.19.530. [DOI] [PubMed] [Google Scholar]
- 42.Serizawa F, Ito K, Matsubara M, et al. Extracorporeal shock wave therapy induces therapeutic lymphangiogenesis in a rat model of secondary lymphoedema. Eur J Vasc Endovasc Surg. 2011;42:254–260. doi: 10.1016/j.ejvs.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 43.Ko DS, Lerner R, Klose G, et al. Effective treatment of lymphedema of the extremities. Arch Surg. 1998;133:452–458. doi: 10.1001/archsurg.133.4.452. [DOI] [PubMed] [Google Scholar]
- 44.Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 45.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82:673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- 46.Mäkinen T, Jussila L, Veikkola T, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 47.Ancukiewicz M, Russell TA, Otoole J, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:1436–1443. doi: 10.1016/j.ijrobp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan Y. Basic fibroblast growth factor-mediated lymphangiogenesis of lymphatic endothelial cells isolated from dog thoracic ducts: effects of heparin. Jpn J Physiol. 1998;48:133–141. doi: 10.2170/jjphysiol.48.133. [DOI] [PubMed] [Google Scholar]
- 49.Shin JW, Min M, Larrieu-Lahargue F, et al. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17:576–584. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dailey L, Ambrosetti D, Mansukhani A, et al. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Sharma R, Wang W, Rasmussen JC, et al. Quantitative imaging of lymph function. Am J Physiol Heart Circ Physiol. 2007;292:H3109–H3118. doi: 10.1152/ajpheart.01223.2006. [DOI] [PubMed] [Google Scholar]
- 52.Kwon S, Sevick-Muraca EM. Noninvasive quantitative imaging of lymph function in mice. Lymphat Res Biol. 2007;5:219–231. doi: 10.1089/lrb.2007.1013. [DOI] [PubMed] [Google Scholar]
- 53.Sharma R, Wendt JA, Rasmussen JC, et al. New horizons for imaging lymphatic function. Ann N Y Acad Sci. 2008;1131:13–36. doi: 10.1196/annals.1413.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rasmussen JC, Tan IC, Marshall MV, et al. Lymphatic imaging in humans with near-infrared fluorescence. Curr Opin Biotechnol. 2009;20:74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stacker SA, Achen MG, Jussila L, et al. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 56.Tsunoda S, Nakamura T, Sakurai H, et al. Fibroblast growth factor-2-induced host stroma reaction during initial tumor growth promotes progression of mouse melanoma via vascular endothelial growth factor A-dependent neovascularization. Cancer Sci. 2007;98:541–548. doi: 10.1111/j.1349-7006.2007.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


