Figure 1.
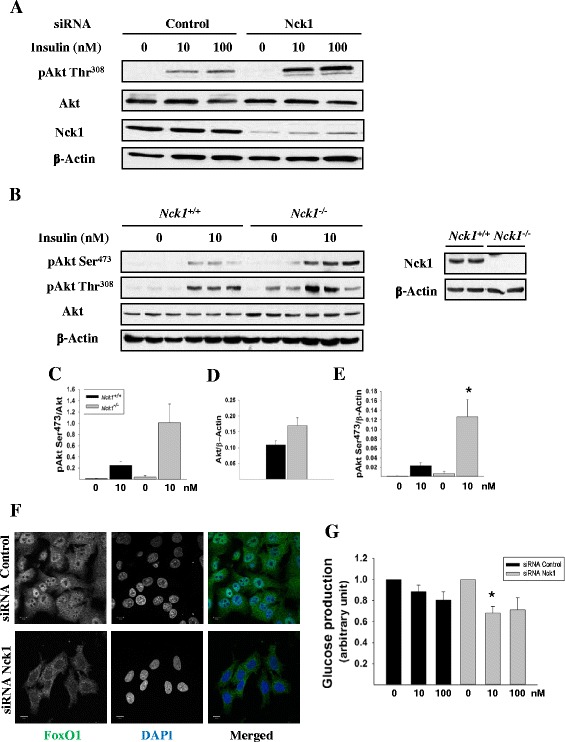
Insulin-induced Akt phosphorylation and downstream signaling are enhanced in Nck1-depleted cells. (A) HepG2 cells transfected with control or Nck1 siRNA were exposed to 0, 10 and 100 nM insulin for 5 min after an overnight serum starvation. Equal amount of proteins from total cell lysates were subjected to immunoblot with indicated antibodies. (B) Primary hepatocytes isolated from Nck1 +/+ and Nck1 −/− mice were left untreated or treated with 10 nM insulin for 5 min. Equal amount of proteins from total cell lysates were subjected to immunoblot with indicated antibodies. Bar charts represent mean ± SEM of the ratio of pAkt/total Akt (C), Akt/β-Actin (D) or pAkt/β-Actin (E) quantified by densitometry analysis. Data are representative of three independent experiments with similar results. *P <0.05 versus Nck1 +/+. (F) Representative confocal images of control or Nck1 siRNA-transfected HepG2 cells stained with FoxO1 antibody and DAPI. Images acquired at 63X magnification are representative of three independent experiments with similar results. Scale bars equal 10 μm. (G) HepG2 cells transfected with control or Nck1 siRNA were exposed to 0, 10 and 100 nM insulin and analyzed for glucose production by measuring glucose levels in glucose-free DMEM. Glucose production was normalized according to protein contents and expressed as relative value compared to basal. Bar chart represents mean ± SEM from six independent experiments performed in triplicate. *P <0.05 versus siRNA control.
