Figure 7.
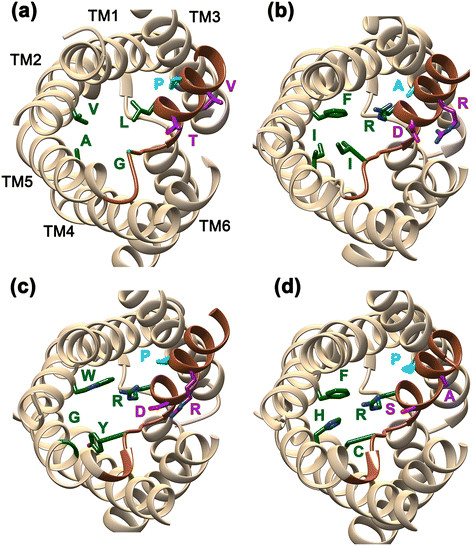
Intra-helical salt-bridge in the loop E half-helix of fungal AQGPs. MIP channels from representative members from (a) SIP-like, (b) δ AQGP, (c) α AQGP and (d) orthodox AQP clusters. The view is down the channel axis from the extracellular side. The residues forming the selectivity filter are shown in green in stick representation. Loop E is shown in dark brown. Acidic and basic residues forming the intra-helical salt-bridge are shown for the δ and α members. The equivalent positions are shown in SIP-like and orthodox AQP members. With the exception of the δ-subgroup, a proline residue occurs at the intervening positions of the acidic and basic residues and it is highly conserved in all other AQGP subgroups and also in most of the orthodox AQPs. This residue is shown in blue and it is substituted by Ala in the representative δ MIP model shown here.
