Summary
Brain metastases are increasingly common tumours but little is known about their biological mechanisms of local brain invasion in vivo. Samples across the leading edge are not easy to obtain using conventional neurosurgical methods as these tumours frequently show cysts, haemorrhage and necrosis. Apparent diffusion coefficient (ADC) maps were generated following diffusion-weighted imaging at 3T using b-values of 0 and 1000 second/mm2 and fused with a 1 mm slice fast spoiled gradient echo sequence. This fused image was used to guide intraoperative biopsies. The technique was performed in ten cases with excellent sampling of the brain-to-metastasis interface, gross total resection and no additional morbidity. Fusing ADC maps with structural scans for intraoperative neuronavigation is a useful method for sampling the leading edge of brain metastases.
Keywords: brain metastasis, diffusion MRI, DWI, ADC, neurosurgery
Introduction
Brain metastases are an increasingly common clinical problem but little is known about their mechanisms of invasion in the human brain. In order to examine local brain invasion by cerebral metastases, samples were acquired from the brain-tumour interface in the course of traditional clinical resection. The quality of samples from routine image-guided neurosurgery using only a post-contrast T1-weighted sequence, however, was not satisfactory with large areas of necrotic cystic deterioration and haemorrhage.
Technical Report
All patients were undergoing gross total resection of a brain metastasis as part of routine clinical care.
Ethical approval was granted as an internal project within the institution's research tissue bank for which all patients give informed consent prior to inclusion (National Research Ethics Service # 11/WNo03/2) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Pre-operative MRI scans were taken at 3T on a Philips Achieva scanner with standard head coils. The imaging included diffusion-weighted imaging (DWI) using single-shot echo planar imaging with two b values of 0 and 1000 second/mm2 (128×128, TR 2828 ms, TE 73 ms, 5 mm slice thickness). Apparent diffusion coefficient (ADC) trace maps were calculated using the post processing software package Philips Extended MR workspace version 2.6.3.1 (Philips Medical Systems, Netherlands). All patients had also undergone a fast spoiled gradient echo sequence with gadolinium contrast (TR/TE 9/1.4, flip angle 15 degrees, 256×256, 180×1 mm slices). These sequences were fused on the StealthStation S7 planning software (Medtronic Inc., MN, USA) and exported to an intraoperative workstation (Figure 1). The fused ADC sequence displayed as a colour map was used to avoid areas of cyst, necrosis which would not have been visible on the standard post-contrast T1 weighted sequence alone and the change in ADC was an indicator of cellular tumour “leading edge” transition to surrounding vasogenic oedema, with the latter having a very high ADC.
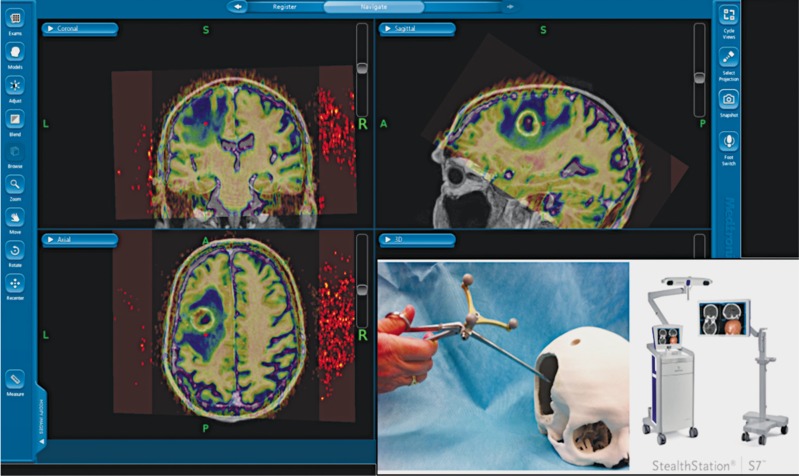
Figure 1.
Intraoperative screenshot of the fused ADC map (displayed as a colour image with high ADC in blue and low ADC in yellow) and inset, the Suretrak© probe attached to a biopsy forceps for use with the StealthStation S7©.
Using these fused sequences, samples were taken using the Suretrak© probe mounted on biopsy forceps. This technique allowed excellent specimens to be taken, demonstrating the transition from tumour to surrounding brain (Figure 2) and allowing greater investigation of metastatic invasion biologically. Post-operative CT brain scan was satisfactory and there was no additional surgical morbidity such as haemorrhage or neurological deficit seen. This method has been applied in ten cases with good results.
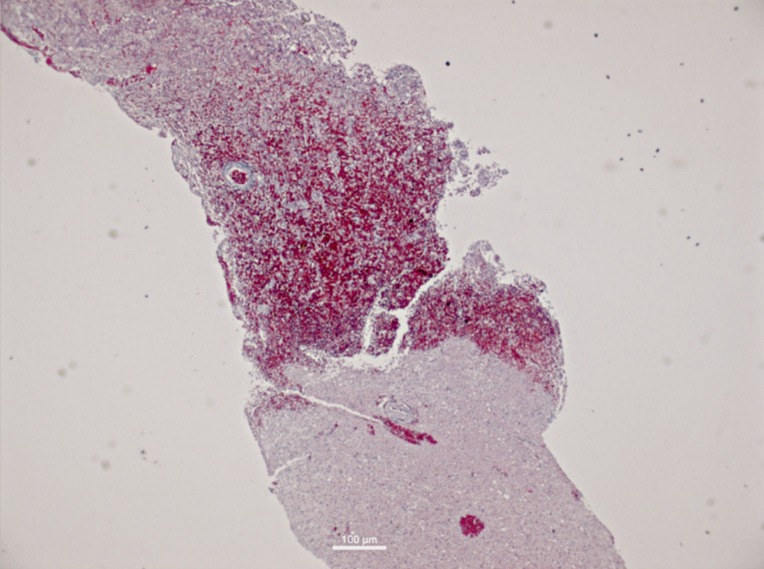
Figure 2.
Haematoxylin and eosin stained section of biopsy taken at the tumour-brain border using the ADC map and intraoperative fusion, showing the transition into peritumoral brain.
Discussion
Previous studies have reported the use of DTI and fMRI sequences intraoperatively in the resection of brain metastases by neuronavigation 1,2 and other sequences such as spectroscopy and PET MRI have been used to guide sampling in glioma 3. We are not aware of ADC maps being used for this specific purpose previously in brain metastases. The effectiveness of ADC maps with colour coding in this series is likely due to the extensive vasogenic oedema seen around brain metastases, providing contrast with the cellular leading edge of the tumour. Meaningful DWI sequences could not be obtained in the presence of significant haemorrhage in two cases and this could limit applicability as metastases from certain primaries (e.g. renal carcinoma) are known to be haemorrhagic.
Conclusions
We present a simple application of existing technology to solve a clinical research problem. Diffusion MRI is a powerful technique for assessing brain tumours and their interaction with surrounding brain and so-called “advanced MR” techniques can be integrated with current image guidance technology to assist neurosurgical procedures.
Acknowledgments
RZ is supported by a Clinical Research Fellowship grant (MR/L017342/1) from the Medical Research Council, UK and research capacity funding from the Walton Centre NHS Foundation Trust, Liverpool, UK.
References
- 1.Hlatky R, Jackson EF, Weinberg JS, et al. Intraoperative neuronavigation using diffusion tensor MR tractography for the resection of a deep tumor adjacent to the corticospinal tract. Stereotact Funct Neurosurg. 2005;83(5-6):228–232. doi: 10.1159/000091954. doi: 10.1159/000091954. [DOI] [PubMed] [Google Scholar]
- 2.Gumprecht H, Ebel GK, Auer DP, et al. Neuronavigation and functional MRI for surgery in patients with lesion in eloquent brain areas. Minim Invasive Neurosurg. 2002;45(3):151–153. doi: 10.1055/s-2002-34341. doi: 10.1055/s-2002-34341. [DOI] [PubMed] [Google Scholar]
- 3.Roessler K, Gatterbauer B, Becherer A, et al. Surgical target selection in cerebral glioma surgery: linking methionine (MET) PET image fusion and neuronavigation. Minim Invasive Neurosurg. 2007;50(5):273–280. doi: 10.1055/s-2007-991143. doi: 10.1055/s-2007-991143. [DOI] [PubMed] [Google Scholar]