Abstract
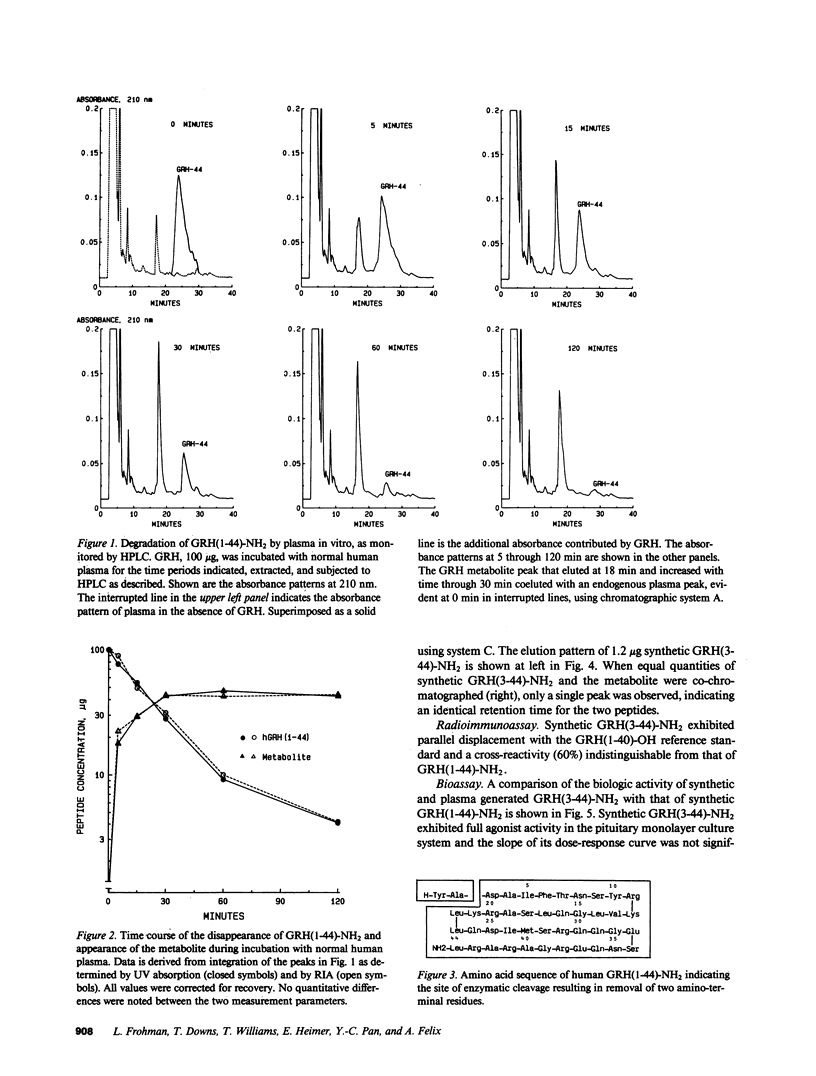
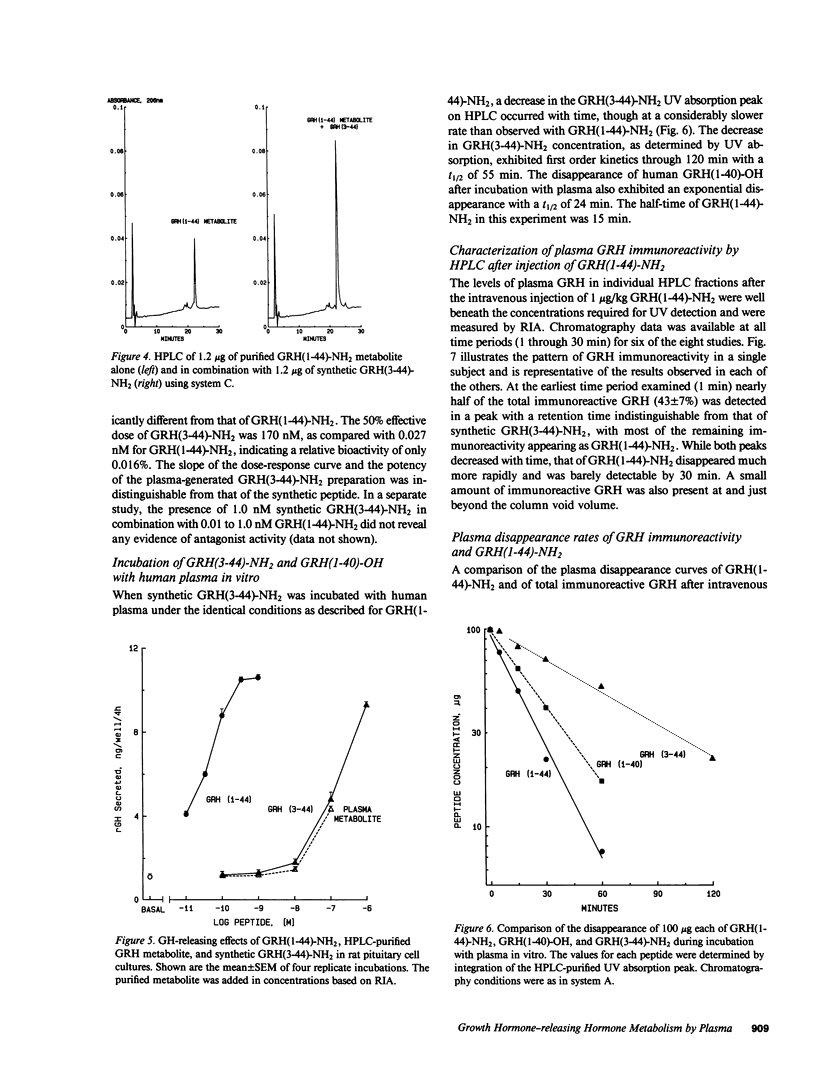
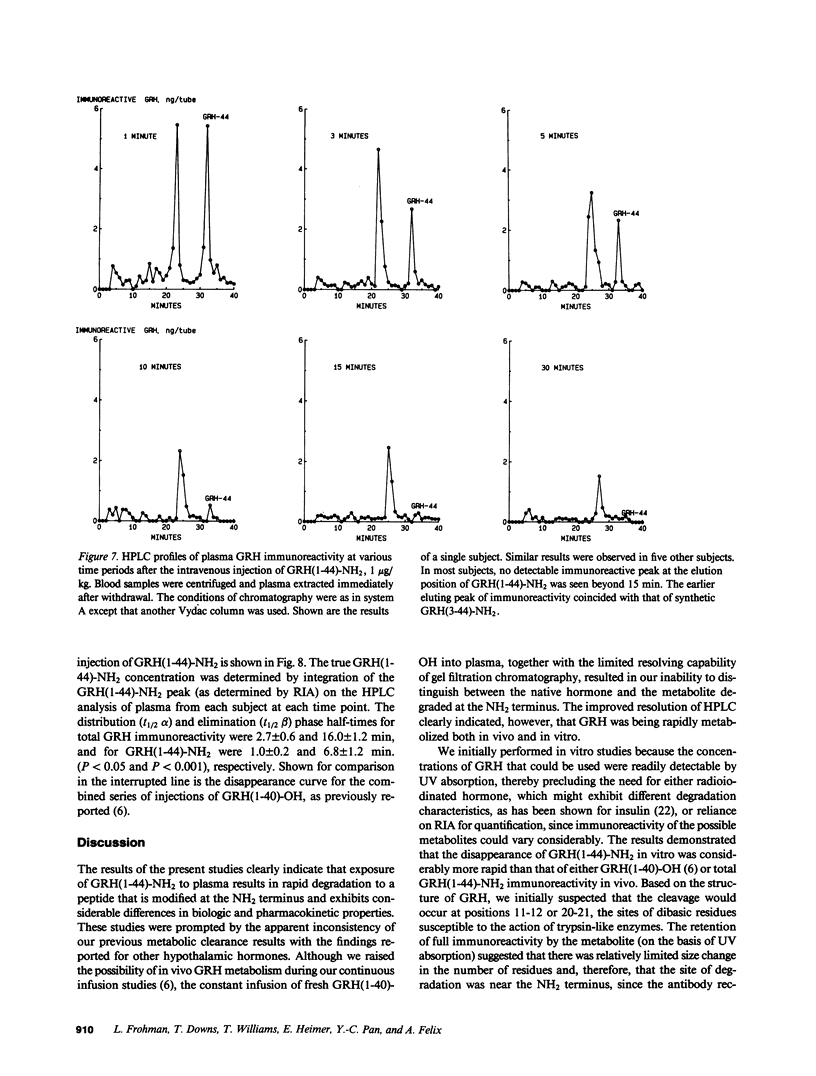
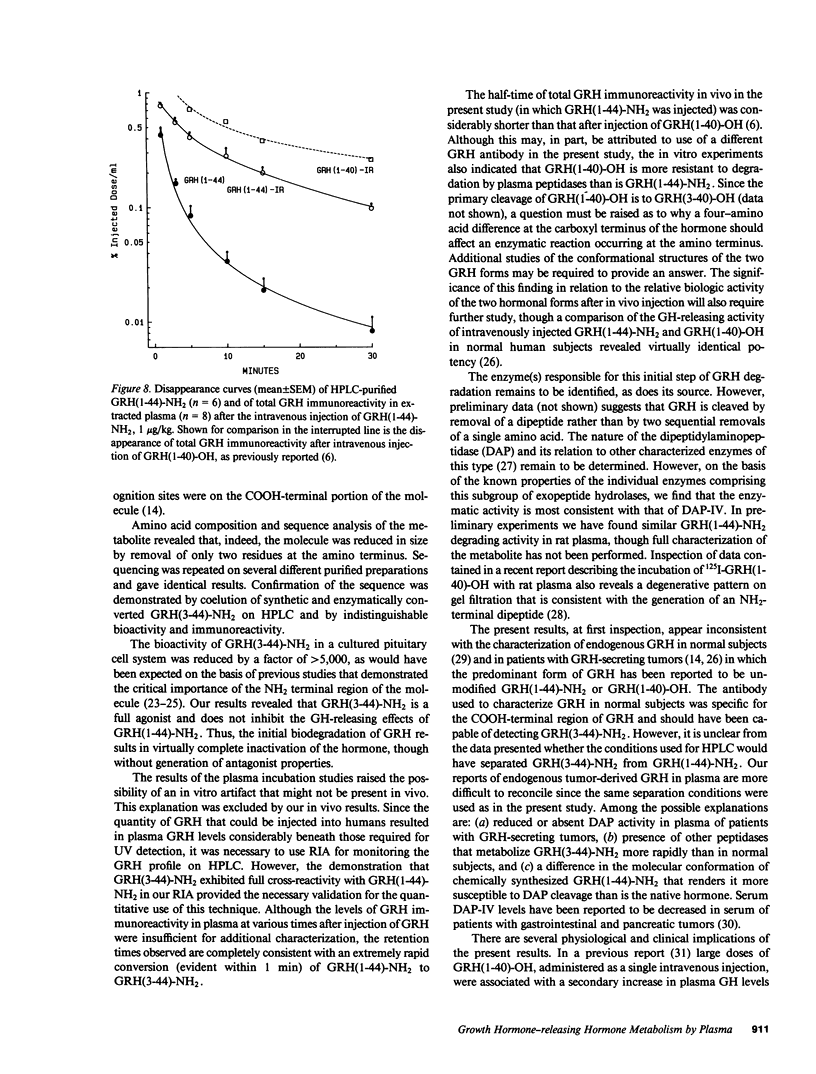
The effect of plasma on degradation of human growth hormone-releasing hormone (GRH) was examined in vitro and in vivo using high performance liquid chromatography (HPLC), radioimmunoassay (RIA), and bioassay. When GRH(1-44)-NH2 was incubated with human plasma, the t1/2 of total GRH immunoreactivity was 63 min (RIA). However, HPLC revealed a more rapid disappearance (t1/2, 17 min) of GRH(1-44)-NH2 that was associated with the appearance of a less hydrophobic but relatively stable peptide that was fully immunoreactive. Sequence analysis indicated its structure to be GRH(3-44)-NH2. Identity was also confirmed by co-elution of purified and synthetic peptides on HPLC. Biologic activity of GRH(3-44)-NH2 was less than 10(-3) that of GRH(1-44)-NH2. After intravenous injection of GRH(1-44)-NH2 in normal subjects, a plasma immunoreactive peak with HPLC retention comparable to GRH(3-44)-NH2 was detected within 1 min and the t1/2 of GRH(1-44)-NH2 (HPLC) was 6.8 min. The results provide evidence for GRH inactivation by a plasma dipeptidylaminopeptidase that could limit its effect on the pituitary.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chihara K., Kashio Y., Kita T., Okimura Y., Kaji H., Abe H., Fujita T. L-dopa stimulates release of hypothalamic growth hormone-releasing hormone in humans. J Clin Endocrinol Metab. 1986 Mar;62(3):466–473. doi: 10.1210/jcem-62-3-466. [DOI] [PubMed] [Google Scholar]
- Coy D. H., Murphy W. A., Sueiras-Diaz J., Coy E. J., Lance V. A. Structure-activity studies on the N-terminal region of growth hormone releasing factor. J Med Chem. 1985 Feb;28(2):181–185. doi: 10.1021/jm00380a006. [DOI] [PubMed] [Google Scholar]
- Evans W. S., Vance M. L., Kaiser D. L., Sellers R. P., Borges J. L., Downs T. R., Frohman L. A., Rivier J., Vale W., Thorner M. O. Effects of intravenous, subcutaneous, and intranasal administration of growth hormone (GH)-releasing hormone-40 on serum GH concentrations in normal men. J Clin Endocrinol Metab. 1985 Nov;61(5):846–850. doi: 10.1210/jcem-61-5-846. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Bernardis L. L. Growth hormone and insulin levels in weanling rats with ventromedial hypothalamic lesions. Endocrinology. 1968 Jun;82(6):1125–1132. doi: 10.1210/endo-82-6-1125. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Downs T. R. Measurement of growth hormone-releasing factor. Methods Enzymol. 1986;124:371–389. doi: 10.1016/0076-6879(86)24029-x. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Jansson J. O. Growth hormone-releasing hormone. Endocr Rev. 1986 Aug;7(3):223–253. doi: 10.1210/edrv-7-3-223. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Szabo M., Berelowitz M., Stachura M. E. Partial purification and characterization of a peptide with growth hormone-releasing activity from extrapituitary tumors in patients with acromegaly. J Clin Invest. 1980 Jan;65(1):43–54. doi: 10.1172/JCI109658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman L. A., Thominet J. L., Webb C. B., Vance M. L., Uderman H., Rivier J., Vale W., Thorner M. O. Metabolic clearance and plasma disappearance rates of human pancreatic tumor growth hormone releasing factor in man. J Clin Invest. 1984 May;73(5):1304–1311. doi: 10.1172/JCI111333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genuth S. M. Metabolic clearance of insulin in man. Diabetes. 1972 Oct;21(10):1003–1012. doi: 10.2337/diab.21.10.1003. [DOI] [PubMed] [Google Scholar]
- Gubler U., Monahan J. J., Lomedico P. T., Bhatt R. S., Collier K. J., Hoffman B. J., Böhlen P., Esch F., Ling N., Zeytin F. Cloning and sequence analysis of cDNA for the precursor of human growth hormone-releasing factor, somatocrinin. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4311–4314. doi: 10.1073/pnas.80.14.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin R., Brazeau P., Böhlen P., Esch F., Ling N., Wehrenberg W. B. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982 Nov 5;218(4572):585–587. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- Hawke D., Yuan P. M., Shively J. E. Microsequence analysis of peptides and proteins. II. Separation of amino acid phenylthiohydantoin derivatives by high-performance liquid chromatography on octadecylsilane supports. Anal Biochem. 1982 Mar 1;120(2):302–311. doi: 10.1016/0003-2697(82)90351-7. [DOI] [PubMed] [Google Scholar]
- Hino M., Nagatsu T., Kakumu S., Okuyama S., Yoshii Y., Nagatsu I. Glycylprolyl beta-naphthylamidase activity in human serum. Clin Chim Acta. 1975 Jul 9;62(1):5–11. doi: 10.1016/0009-8981(75)90273-9. [DOI] [PubMed] [Google Scholar]
- Lance V. A., Murphy W. A., Sueiras-Diaz J., Coy D. H. Super-active analogs of growth hormone-releasing factor (1-29)-amide. Biochem Biophys Res Commun. 1984 Feb 29;119(1):265–272. doi: 10.1016/0006-291x(84)91647-4. [DOI] [PubMed] [Google Scholar]
- Leppaluoto J., Virkkunen P., Lybeck H. Elimination of TRH in man. J Clin Endocrinol Metab. 1972 Sep;35(3):477–478. doi: 10.1210/jcem-35-3-477. [DOI] [PubMed] [Google Scholar]
- Ling N., Baird A., Wehrenberg W. B., Ueno N., Munegumi T., Chiang T. C., Regno M., Brazeau P. Synthesis and in vitro bioactivity of human growth hormone-releasing factor analogs substituted at position-1. Biochem Biophys Res Commun. 1984 Jul 18;122(1):304–310. doi: 10.1016/0006-291x(84)90475-3. [DOI] [PubMed] [Google Scholar]
- Ling N., Esch F., Böhlen P., Brazeau P., Wehrenberg W. B., Guillemin R. Isolation, primary structure, and synthesis of human hypothalamic somatocrinin: growth hormone-releasing factor. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4302–4306. doi: 10.1073/pnas.81.14.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa M., Bock L., Schopohl J., Stalla G. K., Müller O. A., von Werder K. Growth hormone releasing factor infusion does not sustain elevated GH-levels in normal subjects. Acta Endocrinol (Copenh) 1984 Dec;107(4):462–470. doi: 10.1530/acta.0.1070462. [DOI] [PubMed] [Google Scholar]
- Matsuyama H., Ruhmann-Wennhold A., Johnson L. R., Nelson D. H. Disappearance rates of exogenous and endogenous ACTH from rat plasma measured by bioassay and radioimmunoassay. Metabolism. 1972 Jan;21(1):30–35. doi: 10.1016/0026-0495(72)90017-0. [DOI] [PubMed] [Google Scholar]
- Nicholson W. E., DeCherney G. S., Jackson R. V., DeBold C. R., Uderman H., Alexander A. N., Rivier J., Vale W., Orth D. N. Plasma distribution, disappearance half-time, metabolic clearance rate, and degradation of synthetic ovine corticotropin-releasing factor in man. J Clin Endocrinol Metab. 1983 Dec;57(6):1263–1269. doi: 10.1210/jcem-57-6-1263. [DOI] [PubMed] [Google Scholar]
- Pan Y. C., Wideman J., Blacher R., Chang M., Stein S. Use of high-performance liquid chromatography for preparing samples for microsequencing. J Chromatogr. 1984 Aug 3;297:13–19. doi: 10.1016/s0021-9673(01)89024-5. [DOI] [PubMed] [Google Scholar]
- Rafferty B., Schulster D. Radioimmunoassay for human growth hormone-releasing factor (hGRF 1-40): comparison of plasma immunoreactive GRF after intravenous and subcutaneous administration to rats. Mol Cell Endocrinol. 1985 Jun;41(1):19–25. doi: 10.1016/0303-7207(85)90139-x. [DOI] [PubMed] [Google Scholar]
- Redding T. W., Kastin A. J., Gonzales-Barcena D., Coy D. H., Coy E. J., Schalch D. S., Schally A. V. The half-life, metabolism and excretion of tritiated luteinizing hormone-releasing hormone (LH-RH) in man. J Clin Endocrinol Metab. 1973 Oct;37(4):626–631. doi: 10.1210/jcem-37-4-626. [DOI] [PubMed] [Google Scholar]
- Rivier J., Spiess J., Thorner M., Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982 Nov 18;300(5889):276–278. doi: 10.1038/300276a0. [DOI] [PubMed] [Google Scholar]
- Sassolas G., Biot-Laporte S., Cohen R., Elm Charfi A., Ferry S., Borson F. Effects on growth hormone secretion following intravenous and subcutaneous injections of growth hormone-releasing factor (hGRF-44 NH2): comparison of immunoreactive plasma GRF levels. Clin Endocrinol (Oxf) 1985 May;22(5):645–653. doi: 10.1111/j.1365-2265.1985.tb03001.x. [DOI] [PubMed] [Google Scholar]
- Schürmeyer T. H., Avgerinos P. C., Gold P. W., Gallucci W. T., Tomai T. P., Cutler G. B., Jr, Loriaux D. L., Chrousos G. P. Human corticotropin-releasing factor in man: pharmacokinetic properties and dose-response of plasma adrenocorticotropin and cortisol secretion. J Clin Endocrinol Metab. 1984 Dec;59(6):1103–1108. doi: 10.1210/jcem-59-6-1103. [DOI] [PubMed] [Google Scholar]
- Sheppard M., Shapiro B., Pimstone B., Kronheim S., Berelowitz M., Gregory M. Metabolic clearance and plasma half-disappearance time of exogenous somatostatin in man. J Clin Endocrinol Metab. 1979 Jan;48(1):50–53. doi: 10.1210/jcem-48-1-50. [DOI] [PubMed] [Google Scholar]
- Stein S., Brink L. Amino acid analysis of proteins and peptides at the picomole level: the fluorescamine amino acid analyzer. Methods Enzymol. 1981;79(Pt B):20–25. doi: 10.1016/s0076-6879(81)79008-6. [DOI] [PubMed] [Google Scholar]
- Thorner M. O., Vance M. L., Evans W. S., Blizzard R. M., Rogol A. D., Ho K., Leong D. A., Borges J. L., Cronin M. J., MacLeod R. M. Physiological and clinical studies of GRF and GH. Recent Prog Horm Res. 1986;42:589–640. [PubMed] [Google Scholar]
- Vance M. L., Borges J. L., Kaiser D. L., Evans W. S., Furlanetto R., Thominet J. L., Frohman L. A., Rogol A. D., MacLeod R. M., Bloom S. Human pancreatic tumor growth hormone-releasing factor: dose-response relationships in normal man. J Clin Endocrinol Metab. 1984 May;58(5):838–844. doi: 10.1210/jcem-58-5-838. [DOI] [PubMed] [Google Scholar]
- Wilfinger W. W., Larsen W. J., Downs T. R., Wilbur D. L. An in vitro model for studies of intercellular communication in cultured rat anterior pituitary cells. Tissue Cell. 1984;16(4):483–497. doi: 10.1016/0040-8166(84)90026-0. [DOI] [PubMed] [Google Scholar]


