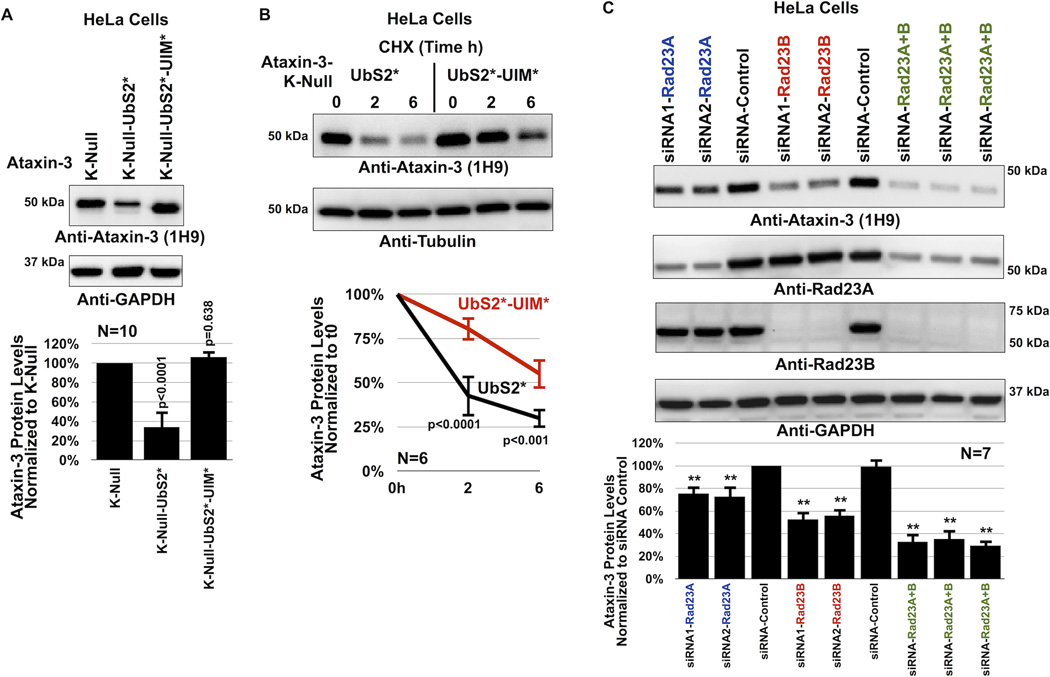
Figure 4. UIMs of ataxin-3 oppose the effect of UbS2 mutation.
A) Top: HeLa cells were transfected as indicated and harvested 48 hours later. Western blots from whole cell lysates. Bottom: Means of ataxin-3 signal quantified from blots on the top and other similar experiments. P values are from ANOVA with Tukey post-hoc correction comparing K-Null ataxin-3 with UbS2 mutated and K-Null ataxin-3 with UbS2 and UIMs mutated to K-Null ataxin-3 with intact domains. Error bars: standard deviations. N=10 independently conducted experiments.
B) Top: HeLa cells were transfected with the indicated constructs. 3× more UbS2* DNA was used than UbS2*-UIM* to begin with approximately the same amount of protein at time 0h. CHX was added to cells 24 hours post transfection for the specified time points. Western blots of whole cell lysates. Bottom: Means of ataxin-3 signal quantified from blots on the top and other similar experiments. P values are from Student T-tests comparing ataxin-3 with UbS2 mutated to ataxin-3 with UbS2 and UIMs mutated. Error bars: standard deviations. N=6 independently conducted experiments.
C) Top: HeLa cells were transfected with the indicated siRNA constructs to knock down endogenous Rad23A, endogenous Rad23B or both, and harvested 48 hours later. Shown are western blots of whole cell lysates. siRNA control: scramble controls. Bottom: Means of ataxin-3 signal quantified from blots on the top and other similar experiments. P values of less than 0.01 are indicated by “**”, and are from ANOVA/Tukey comparing the levels of ataxin-3 protein in RNAi lanes to those in scramble control. Error bars: standard deviations. N=7 independently conducted experiments.