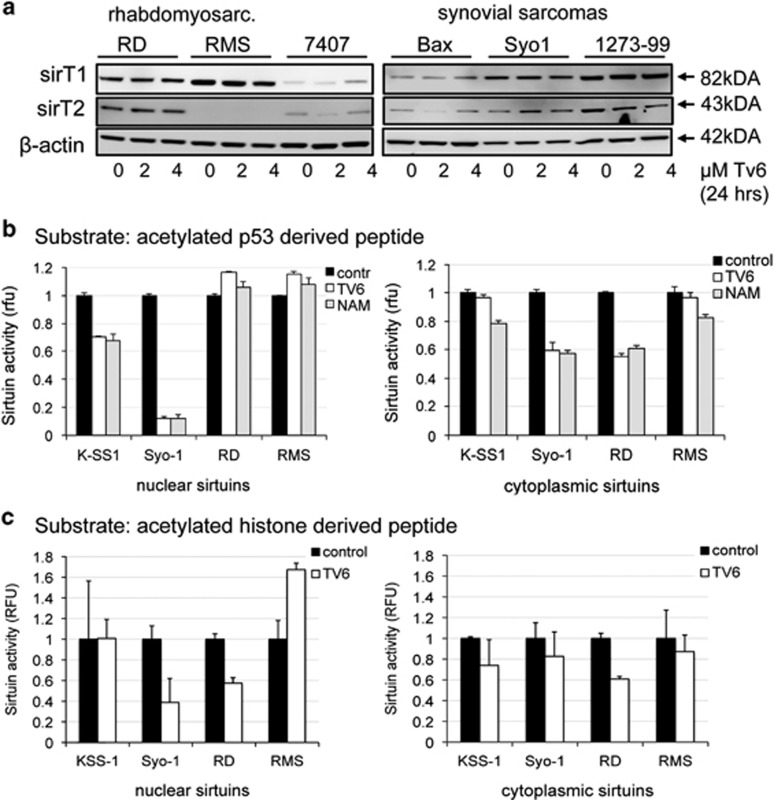
Figure 4.
Tenovin-6 and Nicotinamide (NAM) impair sirtuin deacetylating activity. Synovial sarcoma cell lines (Syo1 and K-SS1) and rhabdomyosarcoma cell lines (RD and RMS) were exposed to tenovin-6 (2 μM and 4 μM) for 24 h, and protein samples were collected to determine sirtuin expression and activity. (a) Western blots showing the expression of SIRT1 and SIRT2 proteins before (0 h) or after exposure to TV6. (b) Sirtuin enzymatic activity in sarcoma cell lines exposed to sirtuin inhibitors. Nuclear- and cytoplasmic-enriched fractions were obtained from control cells and from cells exposed to Tv6 or Nicotinamide (NAM) for 6 h in vivo. Sirtuin activity was determined using a luciferase-based assay that measures deacetylation of a p53-derived peptide (SIRT-GLo Assay, Promega) in the presence of the HDAC inhibitor trichostatin (TSA). The assay measures sirtuin activity using a four-amino-acid acetylated long peptide (QPKK) derived from amino-acid residues 317–320 of p53. The peptide has been conjugated to an aminoluciferin reporting molecule. Experiments were repeated at least three times. (c) Sirtuin enzymatic activity in Tv6-treated sarcoma cell lines using an acetylated histone peptide as substrate. The experiment was performed in the same conditions as in panel b, with the difference that sirtuin activity was determined using a colorimetric assay that measures deacetylation of a histone-derived peptide (Epigenase Universal SIRT Activity/Inhibition Assay, Epigentek) as described in Materials and Methods