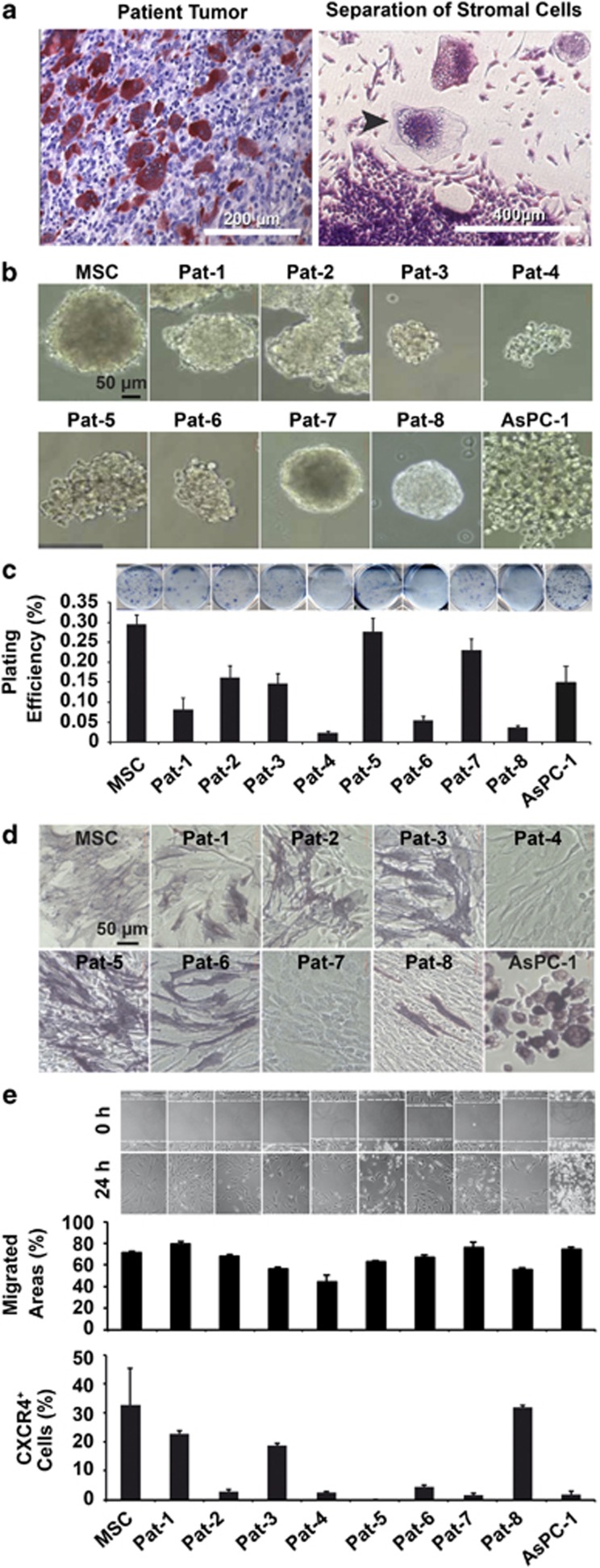
Figure 1.
GCTB stromal cells exhibit self-renewal activity. (a, left) Representative paraffin section out of 20 of a surgically resected GCTB specimen after TRAP staining at × 200 magnification. (Right) Osteoclast-like giant cells and stromal cells in culture after digestion of the tumor tissue. The arrow marks a giant cell surrounded by stromal cells at × 400 magnification. (b) GCTB stromal cells isolated from eight different patient tumors (Pat-1 to Pat-8) were seeded at clonal density in low-adhesion plates. The spheroids were grown until day 7 and photographed at × 100 magnification. MSCs or established pancreatic cancer cells (AsPC-1) served as controls. Data are representative of three independent experiments with similar results. (c) Colony-forming assay of cells plated in medium containing 10% FCS at clonal density of 200 cells/well. Cells were grown without change of medium for 2 weeks, followed by evaluation of fixed and Coomassie blue-stained colonies consisting of at least 50 cells. The plating efficiency as a percentage was calculated using the following formula: 100 × number of colonies/number of seeded cells. Data are presented as the mean of two experiments performed in sextuplicate (n=12)±S.D. (d) The differentiation potential was examined after incubation of cells in osteogenic differentiation medium for 10 days. Alkaline phosphatase expression was detected using BCIP/NBT. Cells were evaluated under × 200 magnification using a Nikon Eclipse TS100 microscope (Nikon Corporation, Sendai, Japan). Data are representative of three independent experiments with similar results. (e) Cells were cultured to 90% confluence before the cell layer was scratched with the tip of a pipette. Closure of the wounded region was evaluated 24 h after scratching by microscopy at × 100 magnification (pictures upper part). CXCR4 expression of GCTB-derived stromal cells was quantified by FACS analysis. Fluorescence intensities±S.D. of eight GCTB stromal patient-derived specimens and controls are shown (diagram lower part). Data are representative of three independent experiments with similar results. Data are representative of three independent experiments with similar results