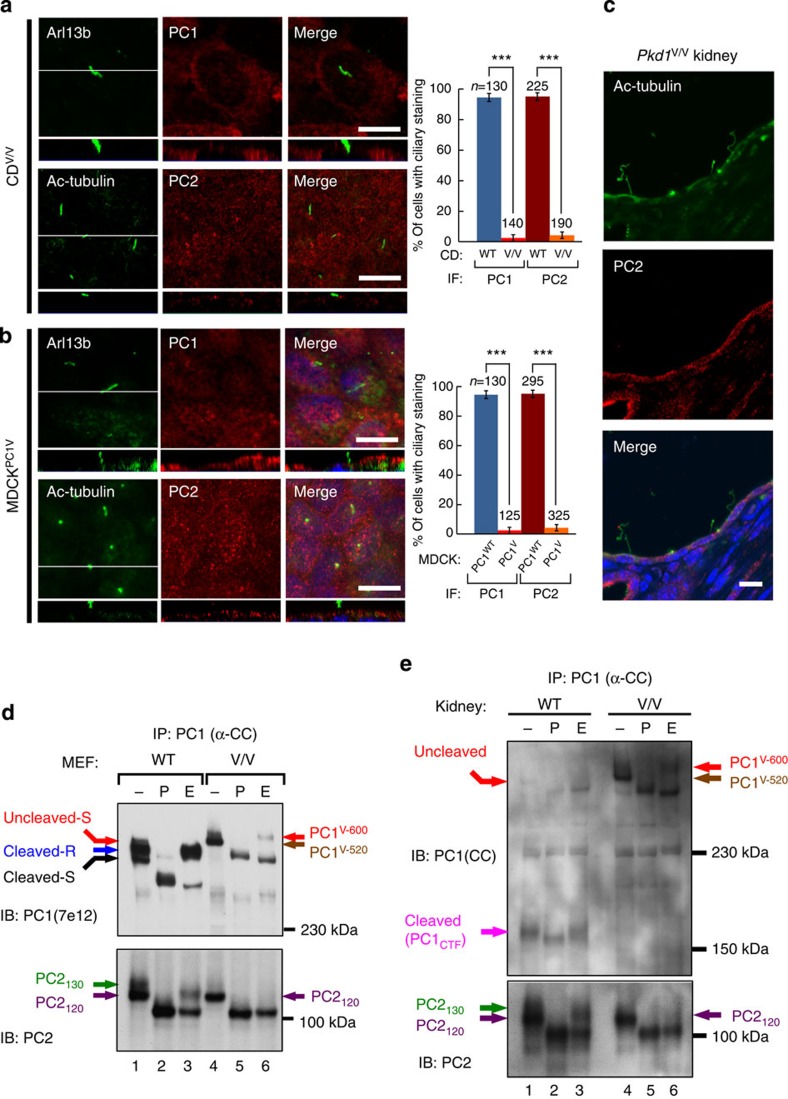
Figure 5. GPS cleavage is required for the ciliary trafficking of polycystin complex.
(a) Confocal images of collecting duct cells isolated from Pkd1V/V kidneys (CDV/V) stained with antibodies against PC1(E4) or PC2. Arl13b or Ac-tubulin was used as a ciliary marker. The graph compares the percentage of cells with positive PC1 or PC2 in the wild-type CD (as shown in Fig. 1e or f) and CDV/V cells, from three independent experiments and presented as the mean±s.e.m.; ***P<0.001. The number of cells analysed (n) is indicated. (b) Confocal images of induced MDCKPC1V cells show the absence of ciliary PC1V and endogenous PC2. The graph compares the percentage of cells with positive PC1 or PC2 ciliary staining in MDCKPC1WT (as shown in Fig. 2f) and MDCKPC1V cells from three independent experiments, presented as the mean±s.e.m.; ***P<0.001. The number of cells analysed (n) is indicated. The white line in the XY scan in (a,b) indicates the path of the XZ scan. Scale bar, 10 μm. (c) Confocal images of cystic collecting ducts of the Pkd1V/V kidney stained with anti-PC2. Note that PC2 was not detectable in 17 of 17 cilia analysed. (d) N-glycosylation pattern of polycystin complex in Pkd1V/V MEFs versus wild-type MEFs. Polycystin complex was immunoprecipitated and analysed as in Fig. 3, with PC1 and PC2 bands indicated with colour code. Note that the co-precipitated PC2 from Pkd1V/V MEFs (lanes 4–6) was entirely EndoH sensitive, lacking EndoH-resistant PC2130 seen in wild-type MEFs (lanes 1–3). (e) N-glycosylation pattern of polycystin complex in Pkd1V/V kidney versus wild-type kidney. Polycystin complex was immunoprecipitated and analysed as in (d), expect that an anti-CC antibody was used to detect PC1. Note the presence of cleaved PC1CTF in wild-type kidney (lanes 1–3) but not in Pkd1V/V kidneys (lanes 4–6).