Abstract
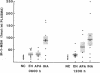
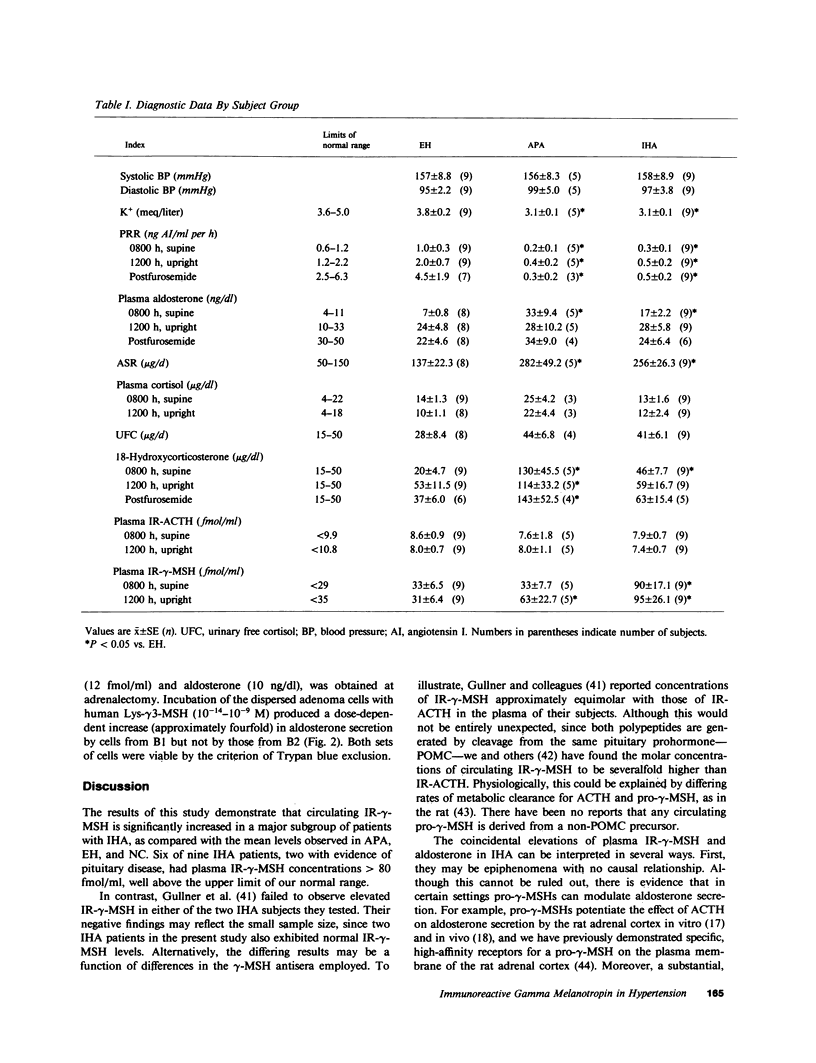
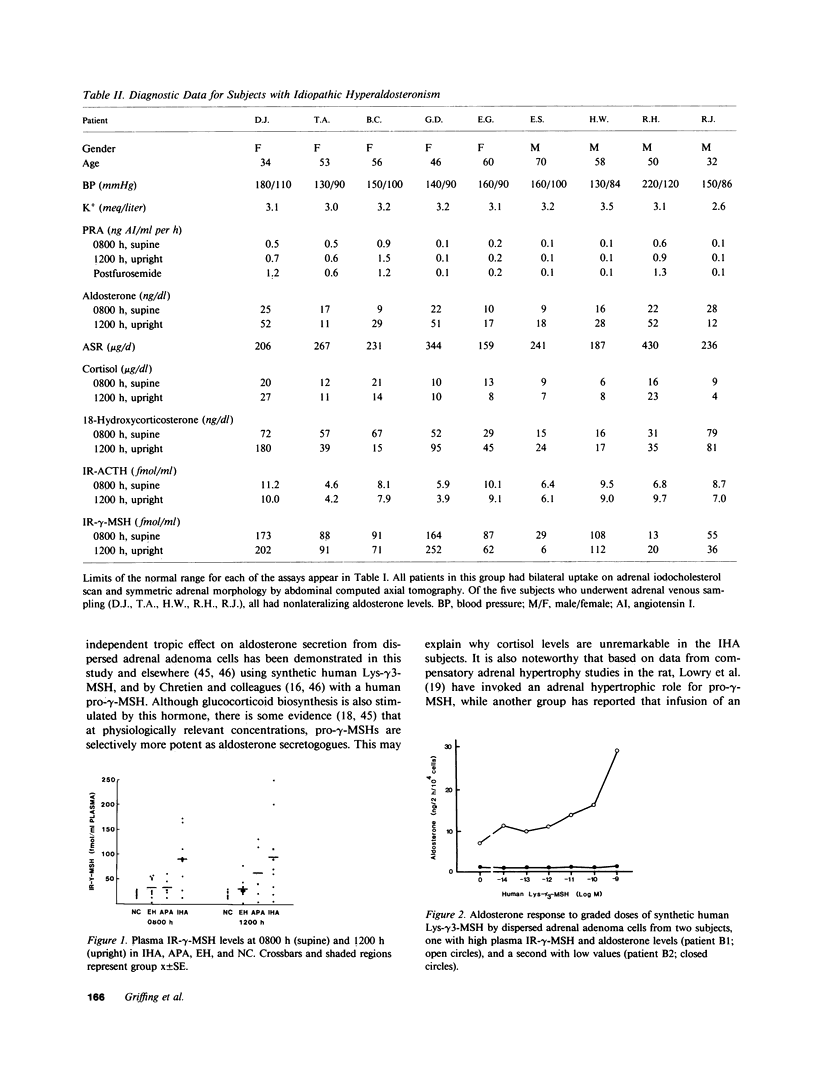
A non-ACTH aldosterone-stimulating factor(s) has been implicated in the pathogenesis of idiopathic hyperaldosteronism (IHA). Although this factor has not been fully characterized, some evidence suggests that it may be related to a pro-gamma-melanotropin (pro-gamma-MSH), derived from the NH2-terminal region of pro-opiomelanocortin. In the present study, plasma immunoreactive (IR-) gamma-MSH levels at 0800 h in patients with IHA were evaluated (90 +/- 17 fmol/ml; range: 13-173 fmol/ml) and found to be significantly higher (P less than 0.05) than those in subjects with aldosterone-producing adenomas (33 +/- 8 fmol/ml), essential hypertension (33 +/- 6 fmol/ml), and normotensive controls (19 +/- 2 fmol/ml). Seven of nine IHA subjects had circulating IR-gamma-MSH levels above the normal range (greater than 35 fmol/ml). In plasmas sampled at 1200 h, IR-gamma-MSH was significantly higher in patients with IHA (95 +/- 26 fmol/ml) and adenomas (63 +/- 23 fmol/ml), as compared with essential hypertensives (31 +/- 6 fmol/ml) and normotensives (19 +/- 3 fmol/ml). Mean plasma IR-ACTH, plasma cortisol, and urinary cortisol levels did not differ significantly between any of these groups. In order to evaluate the effect of a pro-gamma-MSH in vitro, adrenal adenoma tissue was obtained from two patients, one with elevated IR-gamma-MSH (61 fmol/ml) and a second with low IR-gamma-MSH (12 fmol/ml). Aldosterone secretion by dispersed adenoma cells from the former, but not the latter, underwent a fourfold dose-dependent (10(-14)-10(-9) M) increase in response to human Lys-gamma 3-MSH. These data suggest that a pro-gamma-MSH may be implicated as a pathogenic factor in a subset of patients with primary aldosteronism, particularly among those differentially diagnosed as having IHA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Dujaili E. A., Hope J., Estivariz F. E., Lowry P. J., Edwards C. R. Circulating human pituitary pro-gamma-melanotropin enhances the adrenal response to ACTH. Nature. 1981 May 14;291(5811):156–159. doi: 10.1038/291156a0. [DOI] [PubMed] [Google Scholar]
- Antunes J. R., Dale S. L., Melby J. C. Simplified radioimmunoassay for aldosterone using antisera to aldosterone-gamma-lactone. Steroids. 1976 Nov;28(5):621–630. doi: 10.1016/0039-128x(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Biglieri E. G., Schambelan M. The significance of elevated levels of plasma 18-hydroxycorticosterone in patients with primary aldosteronism. J Clin Endocrinol Metab. 1979 Jul;49(1):87–91. doi: 10.1210/jcem-49-1-87. [DOI] [PubMed] [Google Scholar]
- Bravo E. L., Tarazi R. C., Dustan H. P., Fouad F. M., Textor S. C., Gifford R. W., Vidt D. G. The changing clinical spectrum of primary aldosteronism. Am J Med. 1983 Apr;74(4):641–651. doi: 10.1016/0002-9343(83)91022-7. [DOI] [PubMed] [Google Scholar]
- Brown R. D., Wisgerhof M., Carpenter P. C., Brown G., Jiang N. S., Kao P., Hegstad R. Adrenal sensitivity to angiotensin II and undiscovered aldosterone stimulating factors in hypertension. J Steroid Biochem. 1979 Jul;11(1C):1043–1050. doi: 10.1016/0022-4731(79)90049-9. [DOI] [PubMed] [Google Scholar]
- Carey R. M., Sen S., Dolan L. M., Malchoff C. D., Bumpus F. M. Idiopathic hyperaldosteronism. A possible role for aldosterone-stimulating factor. N Engl J Med. 1984 Jul 12;311(2):94–100. doi: 10.1056/NEJM198407123110205. [DOI] [PubMed] [Google Scholar]
- Chan J. S., Seidah N. G., Chrétien M. Measurement of N-terminal (1-76) of human proopiomelanocortin in human plasma: correlation with adrenocorticotropin. J Clin Endocrinol Metab. 1983 Apr;56(4):791–796. doi: 10.1210/jcem-56-4-791. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Mather J. P., Morris P. L., Bardin C. W. Expression of pro-opiomelanocortin-like gene in the testis and epididymis. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5672–5675. doi: 10.1073/pnas.81.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. A., Funder J. W., Tracy K., Morgan F. J., Campbell D. J., Lewis P., Hearn M. T. Adrenocorticotropin, beta-endorphin, and beta-lipotropin in normal thyroid and lung: possible implications for ectopic hormone secretion. Endocrinology. 1982 Dec;111(6):2097–2102. doi: 10.1210/endo-111-6-2097. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Espiner E. A., Donald R. A. Aldosterone regulation in primary aldosteronism: influence of salt balance, posture and ACTH. Clin Endocrinol (Oxf) 1980 Mar;12(3):277–286. doi: 10.1111/j.1365-2265.1980.tb02711.x. [DOI] [PubMed] [Google Scholar]
- Estivariz F. E., Hope J., McLean C., Lowry P. J. Purification and characterization of a gamma-melanotropin precursor from frozen human pituitary glands. Biochem J. 1980 Oct 1;191(1):125–132. doi: 10.1042/bj1910125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster L. B., Dunn R. T. Single-antibody technique for radioimmunoassay of cortisol in unextracted serum or plasma. Clin Chem. 1974 Mar;20(3):365–368. [PubMed] [Google Scholar]
- Franco-Saenz R., Mulrow P. J., Kim K. Idiopathic aldosteronism. A possible disease of the intermediate lobe of the pituitary. JAMA. 1984 May 18;251(19):2555–2558. doi: 10.1001/jama.251.19.2555. [DOI] [PubMed] [Google Scholar]
- Freitas J. E., Grekin R. J., Thrall J. H., Gross M. D., Swanson D. P., Beierwaltes W. H. Adrenal imaging with iodomethyl-norcholesterol (I-131) in primary aldosteronism. J Nucl Med. 1979 Jan;20(1):7–10. [PubMed] [Google Scholar]
- Gross M. D., Grekin R. J., Gniadek T. C., Villareal J. Z. Suppression of aldosterone by cyproheptadine in idiopathic aldosteronism. N Engl J Med. 1981 Jul 23;305(4):181–185. doi: 10.1056/NEJM198107233050401. [DOI] [PubMed] [Google Scholar]
- Güllner H. G., Gill J. R., Jr Beta endorphin selectively stimulates aldosterone secretion in hypophysectomized, nephrectomized dogs. J Clin Invest. 1983 Jan;71(1):124–128. doi: 10.1172/JCI110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güllner H. G., Nicholson W. E., Gill J. R., Jr, Orth D. N. Plasma immunoreactive proopiolipomelanocortin-derived peptides in patients with primary hyperaldosteronism, idiopathic hyperaldosteronism with bilateral adrenal hyperplasia, and dexamethasone-suppressible hyperaldosteronism. J Clin Endocrinol Metab. 1983 Apr;56(4):853–855. doi: 10.1210/jcem-56-4-853. [DOI] [PubMed] [Google Scholar]
- Haber E., Koerner T., Page L. B., Kliman B., Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 1969 Oct;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- Hale A. C., Ratter S. J., Tomlin S. J., Lytras N., Besser G. M., Rees L. H. Measurement of immunoreactive gamma-MSH in human plasma. Clin Endocrinol (Oxf) 1984 Aug;21(2):139–148. doi: 10.1111/j.1365-2265.1984.tb03453.x. [DOI] [PubMed] [Google Scholar]
- Kirkendall W. M., Hammond J. J., Thomas J. C., Overturf M. L., Zama A. Prazosin and clonidine for moderately severe hypertension. JAMA. 1978 Dec 1;240(23):2553–2556. [PubMed] [Google Scholar]
- Korobkin M., White E. A., Kressel H. Y., Moss A. A., Montagne J. P. Computed tomography in the diagnosis of adrenal disease. AJR Am J Roentgenol. 1979 Feb;132(2):231–238. doi: 10.2214/ajr.132.2.231. [DOI] [PubMed] [Google Scholar]
- Krozowski Z., Funder J. W. Mineralocorticoid receptors in rat anterior pituitary: toward a redefinition of "mineralocorticoid hormone". Endocrinology. 1981 Oct;109(4):1221–1224. doi: 10.1210/endo-109-4-1221. [DOI] [PubMed] [Google Scholar]
- Li C. H., Ng T. B., Cheng C. H. Melanotropins: aldosterone- and corticosterone-stimulating activity in isolated rat adrenal cells. Int J Pept Protein Res. 1982 Apr;19(4):361–365. doi: 10.1111/j.1399-3011.1982.tb02616.x. [DOI] [PubMed] [Google Scholar]
- Lim A. T., Khalid B. A., Clements J., Funder J. W. Glucocorticoid and mineralocorticoid effects on adrenocorticotropin and beta-endorphin in the adrenalectomized rat. J Clin Invest. 1982 May;69(5):1191–1198. doi: 10.1172/JCI110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N., Ying S., Minick S., Guillemin R. Synthesis and biological activity of four gamma-melanotropin peptides derived from the cryuptic region of the adrenocorticotropin/beta-lipotropin precursor. Life Sci. 1979 Nov 12;25(20):1773–1779. doi: 10.1016/0024-3205(79)90481-8. [DOI] [PubMed] [Google Scholar]
- Lis M., Hamet P., Gutkowska J., Maurice G., Seidah N. G., Larivière N., Chrétien M., Genest J. Effect of N-terminal portion of pro-opiomelanocortin on aldosterone release by human adrenal adenoma in vitro. J Clin Endocrinol Metab. 1981 Jun;52(6):1053–1056. doi: 10.1210/jcem-52-6-1053. [DOI] [PubMed] [Google Scholar]
- Lowry P. J., Silas L., McLean C., Linton E. A., Estivariz F. E. Pro-gamma-melanocyte-stimulating hormone cleavage in adrenal gland undergoing compensatory growth. Nature. 1983 Nov 3;306(5938):70–73. doi: 10.1038/306070a0. [DOI] [PubMed] [Google Scholar]
- Lu C. L., Chan J. S., De Léan A., Chen A., Seidah N. G., Chrétien M. Metabolic clearance rate and half-time disappearance rate of human N-terminal and adrenocorticotropin of pro-opiomelanocortin in the rat: a comparative study. Life Sci. 1983 Dec 26;33(26):2599–2608. doi: 10.1016/0024-3205(83)90343-0. [DOI] [PubMed] [Google Scholar]
- Matsuoka H., Mulrow P. J., Franco-Saenz R., Li C. H. Stimulation of aldosterone production by beta-melanotropin. Nature. 1981 May 14;291(5811):155–156. doi: 10.1038/291155a0. [DOI] [PubMed] [Google Scholar]
- Matsuoka H., Mulrow P. J., Li C. H. Beta-lipotropin: a new aldosterone-stimulating factor. Science. 1980 Jul 11;209(4453):307–308. doi: 10.1126/science.6247763. [DOI] [PubMed] [Google Scholar]
- McAreavey D., Murray G. D., Lever A. F., Robertson J. I. Similarity of idiopathic aldosteronism and essential hypertension. A statistical comparison. Hypertension. 1983 Jan-Feb;5(1):116–121. doi: 10.1161/01.hyp.5.1.116. [DOI] [PubMed] [Google Scholar]
- Melby J. C., Spark R. F., Dale S. L., Egdahl R. H., Kahn P. C. Diagnosis and localization of aldosterone-producing adenomas by adrenal-vein cateterization. N Engl J Med. 1967 Nov 16;277(20):1050–1056. doi: 10.1056/NEJM196711162772002. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Nicholls M. G., Espiner E. A., Hughes H., Ross J., Stewart D. T. Primary aldosteronism. A study in contrasts. Am J Med. 1975 Sep;59(3):334–342. doi: 10.1016/0002-9343(75)90391-5. [DOI] [PubMed] [Google Scholar]
- Nicholson W. E., Davis D. R., Sherrell B. J., Orth D. N. Rapid radioimmunoassay for corticotropin in unextracted human plasma. Clin Chem. 1984 Feb;30(2):259–265. [PubMed] [Google Scholar]
- Orwoll E. S., Kendall J. W. Beta-endorphin and adrenocorticotropin in extrapituitary sites: gastrointestinal tract. Endocrinology. 1980 Aug;107(2):438–442. doi: 10.1210/endo-107-2-438. [DOI] [PubMed] [Google Scholar]
- Padfield P. L., Brown J. J., Davies D., Fraser R., Lever A. F., Morton J. J., Robertson J. I. The myth of idiopathic hyperaldosteronism. Lancet. 1981 Jul 11;2(8237):83–84. doi: 10.1016/s0140-6736(81)90424-4. [DOI] [PubMed] [Google Scholar]
- Pedersen R. C., Brownie A. C., Ling N. Pro-adrenocorticotropin/endorphin-derived peptides: coordinate action on adrenal steroidogenesis. Science. 1980 May 30;208(4447):1044–1046. doi: 10.1126/science.6246578. [DOI] [PubMed] [Google Scholar]
- Pedersen R. C., Brownie A. C. Lys-gamma 3-melanotropin binds with high affinity to the rat adrenal cortex. Endocrinology. 1983 Apr;112(4):1279–1287. doi: 10.1210/endo-112-4-1279. [DOI] [PubMed] [Google Scholar]
- Pedersen R. C., Ling N., Brownie A. C. Immunoreactive gamma-melanotropin in rat pituitary and plasma: a partial characterization. Endocrinology. 1982 Mar;110(3):825–834. doi: 10.1210/endo-110-3-825. [DOI] [PubMed] [Google Scholar]
- Pratt J. H., Holbrook M. M., Dale S. L., Melby J. C. The measurement of urinary tetrahydroaldosterone by radioimmunoassay. J Steroid Biochem. 1977 Jun;8(6):677–681. doi: 10.1016/0022-4731(77)90296-5. [DOI] [PubMed] [Google Scholar]
- Saito I., Bravo E. L., Zanella T., Sen S., Bumpus F. M. Steroidogenic characteristics of a new aldosterone-stimulating factor (ASF) isolated from normal human urine. Hypertension. 1981 May-Jun;3(3):300–305. doi: 10.1161/01.hyp.3.3.300. [DOI] [PubMed] [Google Scholar]
- Schambelan M., Brust N. L., Chang B. C., Slater K. L., Biglieri E. G. Circadian rhythm and effect of posture on plasma aldosterone concentration in primary aldosteronism. J Clin Endocrinol Metab. 1976 Jul;43(1):115–131. doi: 10.1210/jcem-43-1-115. [DOI] [PubMed] [Google Scholar]
- Schiffrin E. L., Chrétien M., Seidah N. G., Lis M., Gutkowska J., Cantin M., Genest J. Response of human aldosteronoma cells in culture to the N-terminal glycopeptide of pro-opiomelanocortin and gamma 3-MSH. Horm Metab Res. 1983 Apr;15(4):181–184. doi: 10.1055/s-2007-1018663. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Rochemont J., Hamelin J., Lis M., Chrétien M. Primary structure of the major human pituitary pro-opiomelanocortin NH2-terminal glycopeptide. Evidence for an aldosterone-stimulating activity. J Biol Chem. 1981 Aug 10;256(15):7977–7984. [PubMed] [Google Scholar]
- Sen S., Bumpus F. M., Oberfield S., New M. I. Development and preliminary application of a new assay for aldosterone stimulating factor. Hypertension. 1983 Mar-Apr;5(2 Pt 2):I27–I31. doi: 10.1161/01.hyp.5.2_pt_2.i27. [DOI] [PubMed] [Google Scholar]
- Sharp B., Sowers J. R. Adrenocortical response to corticotropin is inhibited by gamma 3-MSH antisera in normotensive and spontaneously hypertensive rats. Biochem Biophys Res Commun. 1983 Jan 27;110(2):357–363. doi: 10.1016/0006-291x(83)91156-7. [DOI] [PubMed] [Google Scholar]
- Szalay K. S., Stark E. Effect of beta-endorphin on the steroid production of isolated zona glomerulosa and zona fasciculata cells. Life Sci. 1981 Sep 28;29(13):1355–1361. doi: 10.1016/0024-3205(81)90679-2. [DOI] [PubMed] [Google Scholar]
- Vinson G. P., Whitehouse B. J., Dell A., Etienne T., Morris H. R. Characterisation of an adrenal zona glomerulosa-stimulating component of posterior pituitary extracts as alpha-MSH. Nature. 1980 Apr 3;284(5755):464–467. doi: 10.1038/284464a0. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Washburn D. D., Kem D. C., Orth D. N., Nicholson W. E., Chrétien M., Mount C. D. Effect of beta-lipotropin on aldosterone production in the isolated rat adrenal cell preparation. J Clin Endocrinol Metab. 1982 Mar;54(3):613–618. doi: 10.1210/jcem-54-3-613. [DOI] [PubMed] [Google Scholar]
- Weinberger M. H., Grim C. E., Hollifield J. W., Kem D. C., Ganguly A., Kramer N. J., Yune H. Y., Wellman H., Donohue J. P. Primary aldosteronism: diagnosis, localization, and treatment. Ann Intern Med. 1979 Mar;90(3):386–395. doi: 10.7326/0003-4819-90-3-386. [DOI] [PubMed] [Google Scholar]
- White E. A., Schambelan M., Rost C. R., Biglieri E. G., Moss A. A., Korobkin M. Use of computed tomography in diagnosing the cause of primary aldosteronism. N Engl J Med. 1980 Dec 25;303(26):1503–1507. doi: 10.1056/NEJM198012253032603. [DOI] [PubMed] [Google Scholar]
- Wilkes M. M., Watkins W. B., Stewart R. D., Yen S. S. Localization and quantitation of beta-endorphin in human brain and pituitary. Neuroendocrinology. 1980;30(2):113–121. doi: 10.1159/000122985. [DOI] [PubMed] [Google Scholar]
- Yamakado M., Franco-Saenz R., Mulrow P. J. Effect of sodium deficiency on beta-melanocyte-stimulating hormone stimulation of aldosterone in isolated rat adrenal cells. Endocrinology. 1983 Dec;113(6):2168–2172. doi: 10.1210/endo-113-6-2168. [DOI] [PubMed] [Google Scholar]
- Zavecz J. H., Levi R. Separation of primary and secondary cardiovascular events in systemic anaphylaxis. Circ Res. 1977 Jan;40(1):15–19. doi: 10.1161/01.res.40.1.15. [DOI] [PubMed] [Google Scholar]