Abstract
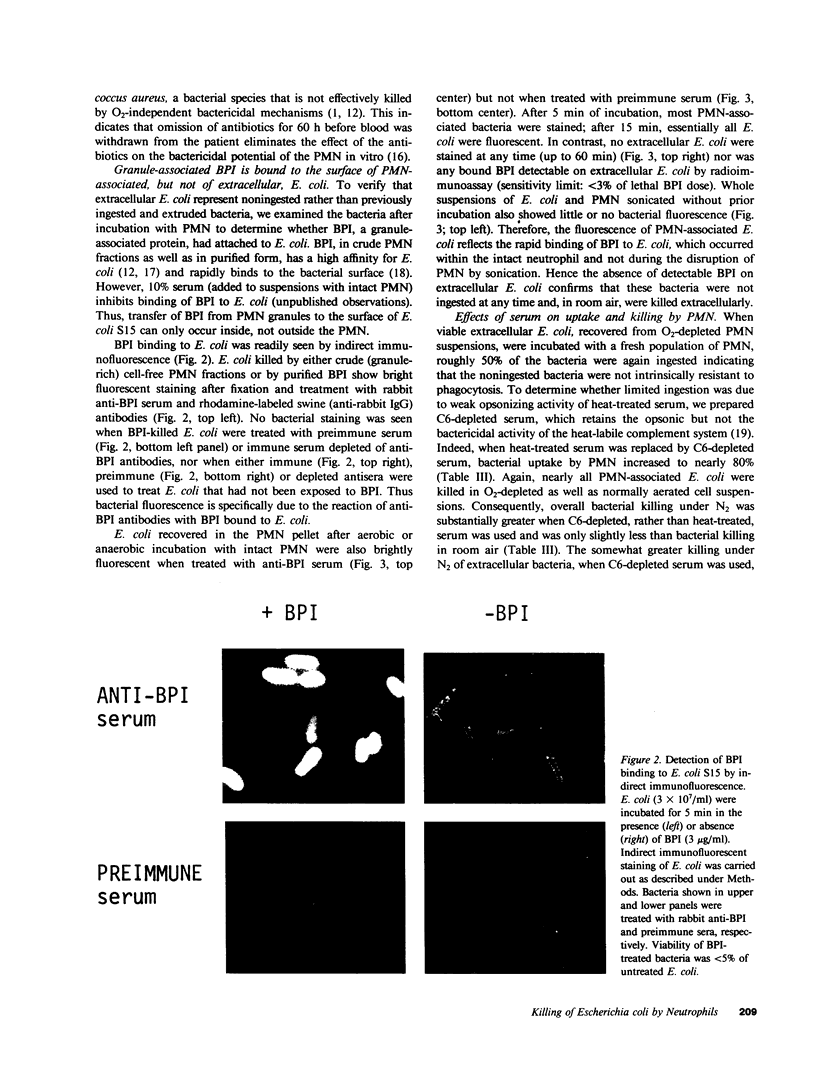
Effective killing of bacteria by polymorphonuclear leukocytes (PMN) is generally assumed to require intracellular sequestration and, depending on the bacterial species, can be both O2-dependent or O2-independent. Killing of several strains of Salmonella typhimurium and Escherichia coli by rabbit PMN does not require O2 and is apparently due to a granule-associated bactericidal/permeability-increasing protein (BPI) present in rabbit and human PMN. In this study we examined the O2 dependence of the killing of E. coli (S15) by human PMN. Ingested and noningested E. coli were separated by centrifugation after incubation with PMN in room air or under N2. In the presence of heat-treated serum approximately 50% of E. coli (10 bacteria/PMN) were taken up by PMN and rapidly (5-15 min) killed both in room air and under N2. The remaining extracellular bacteria (approximately 50%) were killed during 30-60 min of incubation in room air but not under N2. When uptake of E. coli by PMN was increased to approximately 80% by the use of C6-depleted serum (retaining heat-labile opsonins), bacterial survival under N2 was reduced from 54 +/- 7.6% to 13 +/- 5.5%. PMN from a patient with chronic granulomatous disease killed PMN-associated but not extracellular E. coli. BPI was detected, by indirect immunofluorescence, on the surface of PMN-associated E. coli within 5 min of incubation of E. coli with PMN both in room air and under N2. In contrast, at no time was BPI detected on the surface of extracellular E. coli, indicating that the non-PMN-associated E. coli had not been previously ingested. Thus, killing of ingested E. coli S15 by human as well as rabbit PMN does not require O2 and appears to be BPI-mediated. However, when ingestion is limited, extracellular bacteria can also be killed but principally by O2-dependent mechanisms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Pettis P., Beckerdite S., Franson R. Effects of phagocytosis by rabbit granulocytes on macromolecular synthesis and degradation in different species of bacteria. J Bacteriol. 1973 Aug;115(2):490–497. doi: 10.1128/jb.115.2.490-497.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. A reevaluation of the roles of the O2-dependent and O2-independent microbicidal systems of phagocytes. Rev Infect Dis. 1983 Sep-Oct;5(5):843–853. doi: 10.1093/clinids/5.5.843. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Weiss J., Franson R. C., Beckerdite-Quagliata S., Schneider A., Harris L. Separation and purification of a potent bactericidal/permeability-increasing protein and a closely associated phospholipase A2 from rabbit polymorphonuclear leukocytes. Observations on their relationship. J Biol Chem. 1979 Nov 10;254(21):11000–11009. [PubMed] [Google Scholar]
- Mandell G. L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974 Feb;9(2):337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N., Ishibashi S., Takano T. Evidence for bactericidal activity of polymorphonuclear leukocytes without phagocytosis. J Biochem. 1979 Aug;86(2):469–475. doi: 10.1093/oxfordjournals.jbchem.a132546. [DOI] [PubMed] [Google Scholar]
- Okamura N., Spitznagel J. K. Outer membrane mutants of Salmonella typhimurium LT2 have lipopolysaccharide-dependent resistance to the bactericidal activity of anaerobic human neutrophils. Infect Immun. 1982 Jun;36(3):1086–1095. doi: 10.1128/iai.36.3.1086-1095.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passo S. A., Weiss S. J. Oxidative mechanisms utilized by human neutrophils to destroy Escherichia coli. Blood. 1984 Jun;63(6):1361–1368. [PubMed] [Google Scholar]
- Rausch P. G., Pryzwansky K. B., Spitznagel J. K. Immunocytochemical identification of azurophilic and specific granule markers in the giant granules of Chediak-Higashi neutrophils. N Engl J Med. 1978 Mar 30;298(13):693–698. doi: 10.1056/NEJM197803302981301. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- SIMON E. J., VANPRAAG D. INHIBITION OF RNA SYNTHESIS IN ESCHERICHIA COLI BY LEVORPHANOL. Proc Natl Acad Sci U S A. 1964 May;51:877–883. doi: 10.1073/pnas.51.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R. Inborn errors of oxygen-dependent microbial killing by neutrophils. Ergeb Inn Med Kinderheilkd. 1984;51:29–116. doi: 10.1007/978-3-642-69070-9_2. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979 Feb;23(2):522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase-hydrogen peroxide-chloride antimicrobial system: effect of exogenous amines on antibacterial action against Escherichia coli. Infect Immun. 1979 Jul;25(1):110–116. doi: 10.1128/iai.25.1.110-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M., Weiss J., Elsbach P. Heparin inhibits phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1981 Apr;32(1):295–299. doi: 10.1128/iai.32.1.295-299.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Weiss J., Franson R. C., Beckerdite S., Schmeidler K., Elsbach P. Partial characterization and purification of a rabbit granulocyte factor that increases permeability of Escherichia coli. J Clin Invest. 1975 Jan;55(1):33–42. doi: 10.1172/JCI107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Victor M., Elsbach P. Role of charge and hydrophobic interactions in the action of the bactericidal/permeability-increasing protein of neutrophils on gram-negative bacteria. J Clin Invest. 1983 Mar;71(3):540–549. doi: 10.1172/JCI110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Victor M., Stendhal O., Elsbach P. Killing of gram-negative bacteria by polymorphonuclear leukocytes: role of an O2-independent bactericidal system. J Clin Invest. 1982 Apr;69(4):959–970. doi: 10.1172/JCI110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Lampert M. B., Test S. T. Long-lived oxidants generated by human neutrophils: characterization and bioactivity. Science. 1983 Nov 11;222(4624):625–628. doi: 10.1126/science.6635660. [DOI] [PubMed] [Google Scholar]