Abstract
Study Objectives:
Previous studies have associated restless legs syndrome (RLS) with peripheral hypoxia and impaired thermoregulation in the lower extremities. We performed long-term monitoring of skin temperatures in order to investigate whether these findings could be explained by reduced blood flow to the peripheral tissues.
Methods:
96-hour continuous measurements of skin temperature were performed both in the distal and proximal parts of the body of 15 patients with RLS and 14 healthy controls. During the recording, the patients participated in suggested immobilization tests both with and without pramipexole medication.
Results:
We found no baseline differences in distal or proximal skin temperature between patients and controls in daytime or during immobilization. However, pramipexole significantly increased distal skin temperature in the patient group during immobilization (31.1°C vs. 32.9°C, p < 0.05). Daytime temperatures were not affected by therapy or disease status.
Conclusions:
The data suggest that patients with RLS and healthy controls have similar blood flow to the peripheral skin tissue. Pramipexole, however, alters thermoregulation and the previous studies might have been biased by medication. Dopaminergic medication is a major confounding factor when assessing peripheral phenomena in RLS and should be controlled for in the future studies.
Citation:
Salminen AV, Rimpilä V, Polo O. Pramipexole alters thermoregulation in restless legs syndrome. J Clin Sleep Med 2014;10(12):1325-1329.
Keywords: restless legs syndrome, skin temperature, thermoregulation, pramipexole
Restless legs syndrome (RLS), recently known as Willis-Ekbom disease, is associated with discomfort precipitated by immobility in the evening hours.1 Traditionally, RLS is believed to be of central origin, but recently RLS has also been associated with several findings suggesting peripheral abnormalities. Most importantly, a recent study suggested the appearance of peripheral hypoxia during immobilization in patients with RLS.2 In addition, the skeletal muscles of the legs of RLS patients have been shown to have higher capillary tortuosity3 and up-regulated vascular endothelial growth factor (VEGF).4 These findings imply that these patients suffer from peripheral hypoxia. The hypoxia could result from insufficient blood flow to the legs either during daytime or only during immobility. To assess this hypothesis, continuous blood flow measures are warranted in RLS patients.
Continuous measurement of skin blood flow may be achieved by measuring changes in skin temperature. Skin temperatures are commonly measured both distally and proximally, and a distal-to-proximal gradient (DPG) is calculated to assess the blood flow to the distal parts of the body.5 Distal skin temperature rises at sleep onset while the DPG of skin temperature shifts closer to zero, coinciding with the increase in melatonin secretion.6 These data show that DPG is a selective tool for continuously assessing the thermoregulatory blood flow.
Body temperature could be an important factor in RLS. Patients with RLS often complain of feelings of hot or cold temperature in the legs. Idiopathic RLS is associated with impaired perception of temperature resulting from impaired somatosensory processing of the stimulus.7 Abnormal pain responses to heat stimulus have also been reported in RLS.8 Thermal hypoesthesia, on the other hand, is associated with secondary but not idiopathic RLS.9 Recently, it was demonstrated that RLS could be associated with microvascular changes, manifesting as abnormal skin temperatures,10 possibly partly explaining the abnormal thermal sensations. In this study, we wanted to confirm this finding in a 96-hour continuous skin temperature measurement and assess the effect of dopaminergic medication to skin temperatures in RLS.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Recent studies have shown impaired thermoregulation in medicated patients with restless legs syndrome (RLS). We investigated the effect of pramipexole on thermoregulation in RLS.
Study Impact: Our results demonstrate that pramipexole modifies the thermoregulation in patients with RLS at the time when symptoms occur. Dopaminergic therapy should be well controlled for in future studies assessing peripheral blood flow in RLS.
METHODS
Fifteen patients suffering from RLS and fourteen age- and sex-matched controls with no RLS symptoms were recruited to the study. Only idiopathic RLS patients with current pramipexole medication and no significant other medical conditions were included. All RLS patients had previously measured plasma ferritin > 15 μg/L. Control subjects with known RLS in immediate family were excluded.
RLS patients discontinued their pramipexole medication ≥ 2 weeks before starting the measurements. RLS severity, measured with standard IRLSSG scale,11 and sleepiness (Epworth sleepiness scale, ESS) were evaluated 2 weeks after the discontinuation of the medication. The study protocol lasted for 4 full days (96 h) starting in the evening of day one. Pramipexole medication was re-continued on day 3 of the study, at the dose previously found efficient for suppressing RLS symptoms (ranging from 0.25 to 0.5 μg). Control subjects went through the same protocol, with the exception of not having to take any dopaminergic medication.
Skin temperatures were measured continuously for the duration of 4 days with model DS1922L iButtons (Maxim Integrated, San Jose, CA, U.S.). These devices have been validated for measurement of human skin temperature and have accuracy of 0.09°C and precision of 0.05°C.12 Skin temperature was recorded with an interval of 120 sec from the instep of both feet and parasternally from the chest. Mean of the 2 feet was used to evaluate distal skin temperature. DPG of the skin temperatures (DPG = T(foot) – T(chest)) was calculated.
All subjects went through 4 suggested immobilization tests (SIT). They were performed in the first and fourth evening of the study, 2 and 4 h before regular bedtime. SIT is a standardized test to objectively assess RLS symptoms. The patients are asked to stay still in a semi-sitting position for 60 min.13 Every 5 min during the test, subjects are asked to report the subjective level of leg discomfort on a scale from 0 (no discomfort) to 10 (maximal discomfort). The momentary values of skin temperature were recorded with the same interval. Any distracting activity, such as reading or watching TV, was not allowed during the tests.
Daytime skin temperatures were analyzed as mean values in periods of 1 h. Instead of time of day, self-reported time of awakening in the morning was used as a marker to fix individual recordings to a timeframe for comparison. The analysis spanned from 2 h before awakening in the morning until 10 h after awakening. In RLS patient group, mean values for each time block was calculated separately from 2 days with and 2 days without pramipexole medication. For control patients, an average of 4 days was used.
Skin temperatures both during the SIT and during daytime were compared between the subject groups. Skin temperatures between patients and controls were compared at each time point with 2-tailed Mann-Whitney test. Measurements in RLS patients with and without medication were compared with 2-tailed Wilcoxon signed-rank test. Correlations with RLS severity were evaluated with Spearman rank correlation coefficient. All RLS patients were included in the analysis of correlation.
The study was approved by the local ethical committee (Tampere, Finland). All patients signed informed consent before participation to the study.
RESULTS
The 2 patient groups were matched for gender and age. The patient group included 7 and the control group 6 female subjects. The mean ages in the patient and control groups were 57.1 and 56.6 years, respectively. All patients had a previous diagnosis of idiopathic RLS with plasma ferritin values in the normal range. The mean severity of RLS (IRLSSG scale) without pramipexole medication was 23.7 in the RLS patient group. No difference was found in sleepiness between RLS patients and controls (ESS score 6.1 vs. 4.3, p = 0.275, respectively). RLS patients who did not suffer from RLS symptoms 2 weeks after discontinuation of pramipexole (IRLSSG severity < 15, n = 3) were excluded from the analyses.
The continuous skin temperature measurements showed higher distal skin temperatures during the night, close to the level of normal core body temperature (Figure 1). During the day the average temperatures dropped by 4-5°C. Consequently, the DPG was close to zero during the night and dropped during daytime. No statistically significant differences were observed between RLS patients and healthy controls. Pramipexole, taken the previous evening, did not have an effect on the skin temperature during the next day.
Figure 1. The skin temperatures and skin temperature gradients are displayed as averages in each group.

The values are average values during a period of one hour. X-axis shows the hour, zero being self-reported awakening. Subject groups are RLS patients without therapy (open diamond), RLS patients with pramipexole (closed diamond) and healthy controls (closed triangle). All differences between subject groups are nonsignificant.
During SIT, the RLS patients suffered from greater discomfort than the controls (Table 1). The skin temperatures or DPG did not differ between the 2 subject groups (Figure 2). Continuation of pramipexole resolved the discomfort almost entirely in the RLS patient group. Pramipexole also increased the skin temperature in the extremities but not on the chest. This can be seen as a rise in both the distal skin temperature and DPG (Table 1). Average skin temperature values did not correlate with RLS severity (Foot temperature; ρ = -0.110, DPG; ρ = -0.221).
Table 1.
Mean values of skin temperatures during SIT.
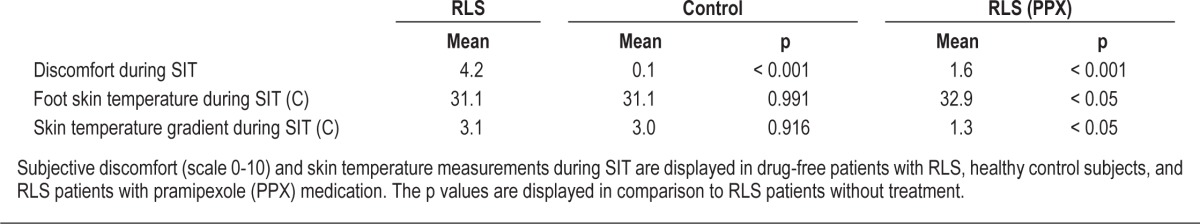
Figure 2. The foot skin temperature and the chest-to-foot gradient of skin temperature at each time point during suggested immobilization.

Subject groups are RLS patients without therapy (open diamond), RLS patients with pramipexole treatment (closed diamond) and healthy controls (closed triangle). * p < 0.05 compared to RLS group without therapy.
DISCUSSION
The original aim of this study was to confirm the previous findings, suggesting impaired microcirculation in the extremities of patients with RLS.2,4 Our 96-hour skin temperature measurements, however, do not support this hypothesis. Skin temperatures remained similar in the patients and controls during daytime and during the provocation of symptoms by SIT. The skin temperatures were, however, increased in RLS patients after administration of pramipexole medication. Therefore, our data suggest that increased distal skin temperatures are associated with dopaminergic treatment rather than RLS.
Skin temperature gradient has been shown to correlate with skin blood flow.6 If the variable is agreed to mirror skin blood flow, our data suggest no difference in blood flow to the skin between RLS patients and healthy controls. On the other hand, dopaminergic medication seems to enhance the blood flow in the peripheral skin tissue. This is supported by previous findings suggesting that dopamine redirects blood flow from the central parts of the body to the peripheral muscles and skin in humans.14 In rats, a D1 and D2 receptor agonist apomorphine reduces the core body temperature,15 further supporting the effect of dopamine on thermoregulation.
Despite the normal baseline blood flow to the peripheral skin tissue demonstrated here, our previous results demonstrated hypoxia specific to the peripheral tissues in RLS patients.2 Signs of hypoxia have also been shown in the peripheral skeletal muscle of patients with RLS.4 This paradox could be explained by reduced oxygen transportation capacity to the periphery. Iron is an important factor in oxygen transport, and therefore could be the underlying reason. Even if our patients had normal serum ferritin levels, changes in iron metabolism have been demonstrated in RLS patients without iron deficiency.16 Alternatively, the hypoxia findings could be related to the symptoms themselves and not to a compromised oxygen transport.
In a recent study, Anderson et al. showed impaired microcirculation in RLS, as demonstrated by laser-Doppler flowmetry and skin temperature measurements.10 However, they enrolled patients with concomitant pramipexole medication. Our data show that the results they obtained are likely to be the effect of pramipexole and not a feature of RLS. Pramipexole increases skin temperature in the legs of RLS patients, while simultaneously resolving the RLS symptoms. This effect of dopaminergic medication on skin temperature is likely to be a result of increased blood flow to the legs and therefore affect also the blood flow measurements by laser-Doppler flowmetry.
The distal skin temperature values correlate with subjective vigilance, as well as with its neurological markers.17,18 This together with our data could suggest that RLS patients are not less vigilant during the daytime or evening hours than healthy controls, despite the sleep loss. This is supported by similar level of sleepiness in the two groups in our study. Previous findings have shown similar results, some even finding higher daytime vigilance in RLS patients than controls.19 After treatment with pramipexole, the patients showed higher distal skin temperatures, possibly indicating reduced vigilance. Indeed, increased daytime somnolence after pramipexole treatment has been demonstrated in Parkinson disease,20 and sleepiness has also been listed as a common side effect of pramipexole in RLS.21
The daytime pattern observed in our study is similar to those reported by previous studies. The pattern of distal skin temperature reaching values of the normal core body temperature during sleep, probably as a consequence of skin vasodilation, has been demonstrated previously.22 Lesser diurnal variation can be seen in skin temperatures on the chest, resulting in a shift in DPG upon awakening. This pattern can also be seen in our data. In our study, both RLS patients and controls, regardless of the status of medication, showed a daytime skin temperature pattern fully comparable to each other and that observed in previous studies in healthy subjects.
RLS patients often report feelings of burning in their legs23 but have normal distal skin temperatures when not on medication, as shown by our data. This implies that the sensations are likely to be explained by decreased thermal pain thresholds or impaired somatosensory processing of the pain signal. Impaired temperature perception has been demonstrated in up to 72% of patients with idiopathic RLS.7 Another study shows increased temporal summation of heat pain in RLS patients compared to healthy controls.8 However, thresholds for thermal perception are not altered in idiopathic RLS.9 Taken together, these data suggest impaired central sensory processing of thermal stimuli in patients with idiopathic RLS, explaining the thermal sensations in the absence of altered skin temperatures.
Our data demonstrate that administration of pramipexole raises the distal skin temperature in patients with RLS. The effect is not visible in the morning hours, as indicated by the longtime measurement of skin temperature (Figure 1), possibly due to the shorter half-life of pramipexole in the body. It remains to be determined if this can be generalized to other dopaminergic therapies or to other types of subjects, including healthy controls. However, previous studies have demonstrated the redirection of blood flow from the central parts of the body to the periphery after dopamine infusion,14 suggesting that the effect could be in common to other dopaminergic therapies, as well as subjects without RLS. Further studies are warranted to confirm the effect of other dopaminergics.
There are limitations to the methods used in our study. The continuous skin temperature measurements were performed at subjects' homes, where the ambient temperature could not be controlled. Another potential source of bias is the placement and number of iButton devices used. In many other studies, the proximal skin temperature is measured with several devices and average values are used. When only one device is used, the site is more likely to be exposed to the ambient temperature. To reduce bias from these sources in daytime recordings, the skin temperatures of patients and matching controls were always recorded simultaneously, in order to avoid bias from the outside weather and ambient temperature. During SIT, the ambient temperature and the clothing of the subjects were controlled.
In conclusion, our data demonstrates normal blood flow to the legs in RLS, despite peripheral hypoxia shown in previous studies. In addition, we show that the previously reported differences in skin temperature and peripheral blood flow could be severely biased by dopaminergic medication. In the future, RLS studies focusing on the peripheral aspects of the disease should be performed in medication-free patients to avoid bias. The absence of differences in distal skin temperatures between RLS patients and controls suggests normal peripheral blood flow in patients with RLS.
DISCLOSURE STATEMENT
This was not an industry supported study. The work was supported by the Tuberculosis Foundation of Tampere, Finland. The work was performed at Unesta Research Centre, Tampere, Finland. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Richard Allen and Dr. Claudia Trenkwalder for help in study design.
REFERENCES
- 1.Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol. 2010;6:337–46. doi: 10.1038/nrneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- 2.Salminen AV, Rimpila V, Polo O. Peripheral hypoxia in restless legs syndrome (Willis-Ekbom disease) Neurology. 2014;82:1856–61. doi: 10.1212/WNL.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 3.Wåhlin-Larsson B, Kadi F, Ulfberg J, Aulin KP. Skeletal muscle morphology in patients with restless legs syndrome. Eur Neurol. 2007;58:133–7. doi: 10.1159/000104712. [DOI] [PubMed] [Google Scholar]
- 4.Wåhlin-Larsson B, Ulfberg J, Aulin KP, Kadi F. The expression of vascular endothelial growth factor in skeletal muscle of patients with sleep disorders. Muscle Nerve. 2009;40:556–61. doi: 10.1002/mus.21357. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein EH, Sessler DI. Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology. 1990;73:541–5. [PubMed] [Google Scholar]
- 6.Krauchi K, Cajochen C, Werth E, Wirz-Justice A. Functional link between distal vasodilation and sleep-onset latency? Am J Physiol Regul Integr Comp Physiol. 2000;278:R741–8. doi: 10.1152/ajpregu.2000.278.3.R741. [DOI] [PubMed] [Google Scholar]
- 7.Schattschneider J, Bode A, Wasner G, Binder A, Deuschl G, Baron R. Idiopathic restless legs syndrome: abnormalities in central somatosensory processing. J Neurol. 2004;251:977–82. doi: 10.1007/s00415-004-0475-3. [DOI] [PubMed] [Google Scholar]
- 8.Edwards RR, Quartana PJ, Allen RP, Greenbaum S, Earley CJ, Smith MT. Alterations in pain responses in treated and untreated patients with restless legs syndrome: associations with sleep disruption. Sleep Med. 2011;12:603–9. doi: 10.1016/j.sleep.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann CG, Rolke R, Scheidt U, et al. Thermal hypoaesthesia differentiates secondary restless legs syndrome associated with small fibre neuropathy from primary restless legs syndrome. Brain. 2010;133:762–70. doi: 10.1093/brain/awq026. [DOI] [PubMed] [Google Scholar]
- 10.Anderson KN, Di Maria C, Allen J. Novel assessment of microvascular changes in idiopathic restless legs syndrome (Willis-Ekbom disease) J Sleep Res. 2013;22:315–21. doi: 10.1111/jsr.12025. [DOI] [PubMed] [Google Scholar]
- 11.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 12.van Marken Lichtenbelt WD, Daanen HA, Wouters L, et al. Evaluation of wireless determination of skin temperature using iButtons. Physiol Behav. 2006;88:489–97. doi: 10.1016/j.physbeh.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Montplaisir J, Boucher S, Nicolas A, et al. Immobilization tests and periodic leg movements in sleep for the diagnosis of restless leg syndrome. Mov Disord. 1998;13:324–9. doi: 10.1002/mds.870130220. [DOI] [PubMed] [Google Scholar]
- 14.Eliasen P, Klemp P, Vagn Nielsen H, Crone P. Subcutaneous and muscle blood flow during dopamine infusion in man. Scand J Clin Lab Invest. 1989;49:43–7. doi: 10.3109/00365518909089076. [DOI] [PubMed] [Google Scholar]
- 15.Faunt JE, Crocker AD. Effects of adrenalectomy on responses mediated by dopamine D-1 and D-2 receptors. Eur J Pharmacol. 1989;162:237–44. doi: 10.1016/0014-2999(89)90286-0. [DOI] [PubMed] [Google Scholar]
- 16.Earley CJ, Ponnuru P, Wang X, et al. Altered iron metabolism in lymphocytes from subjects with restless legs syndrome. Sleep. 2008;31:847–52. doi: 10.1093/sleep/31.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romeijn N, Van Someren EJ. Correlated fluctuations of daytime skin temperature and vigilance. J Biol Rhythms. 2011;26:68–77. doi: 10.1177/0748730410391894. [DOI] [PubMed] [Google Scholar]
- 18.Ramautar JR, Romeijn N, Gomez-Herrero G, Piantoni G, Van Someren EJ. Coupling of infraslow fluctuations in autonomic and central vigilance markers: skin temperature, EEG beta power and ERP P300 latency. Int J Psychophysiol. 2013;89:158–64. doi: 10.1016/j.ijpsycho.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Gamaldo CE, Benbrook AR, Allen RP, Oguntimein O, Earley CJ. A further evaluation of the cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2008;9:500–5. doi: 10.1016/j.sleep.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romigi A, Brusa L, Marciani MG, et al. Sleep episodes and daytime somnolence as result of individual susceptibility to different dopaminergic drugs in a PD patient: a polysomnographic study. J Neurol Sci. 2005;228:7–10. doi: 10.1016/j.jns.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 21.Montplaisir J, Fantini ML, Desautels A, Michaud M, Petit D, Filipini D. Long-term treatment with pramipexole in restless legs syndrome. Eur J Neurol. 2006;13:1306–11. doi: 10.1111/j.1468-1331.2006.01459.x. [DOI] [PubMed] [Google Scholar]
- 22.Krauchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267:R819–29. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- 23.Karroum EG, Golmard JL, Leu-Semenescu S, Arnulf I. Sensations in restless legs syndrome. Sleep Med. 2012;13:402–8. doi: 10.1016/j.sleep.2011.01.021. [DOI] [PubMed] [Google Scholar]


