Abstract
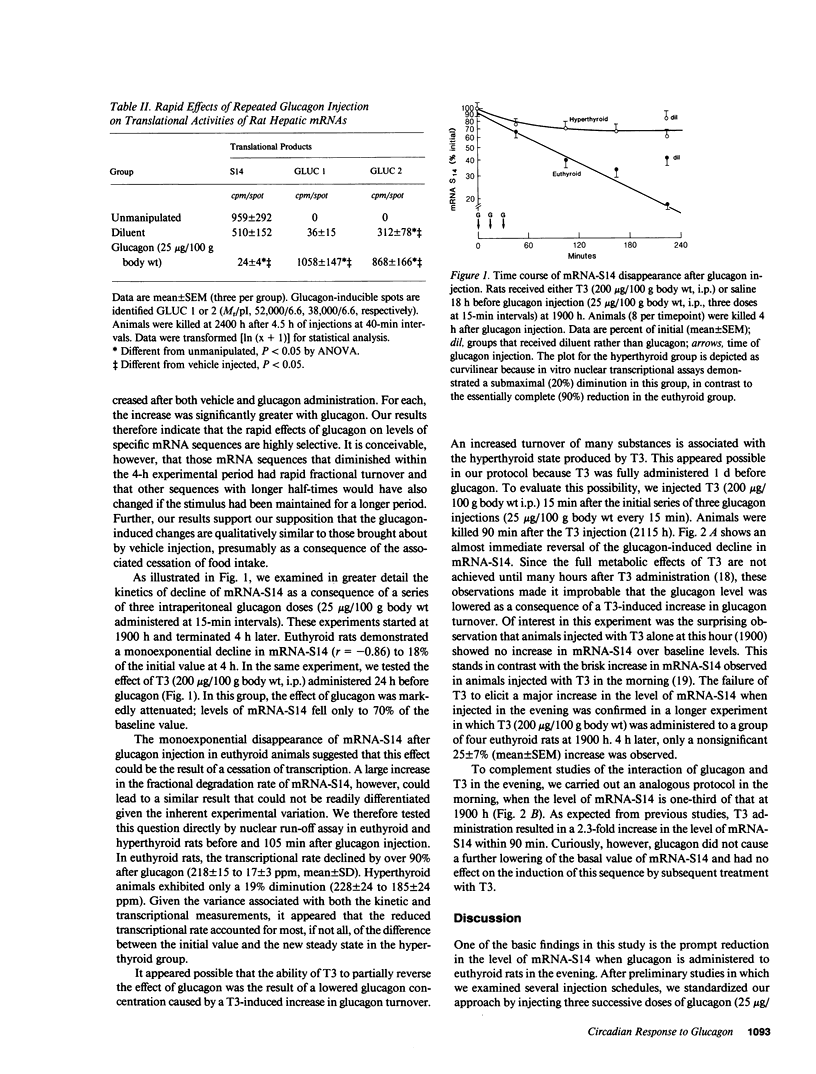
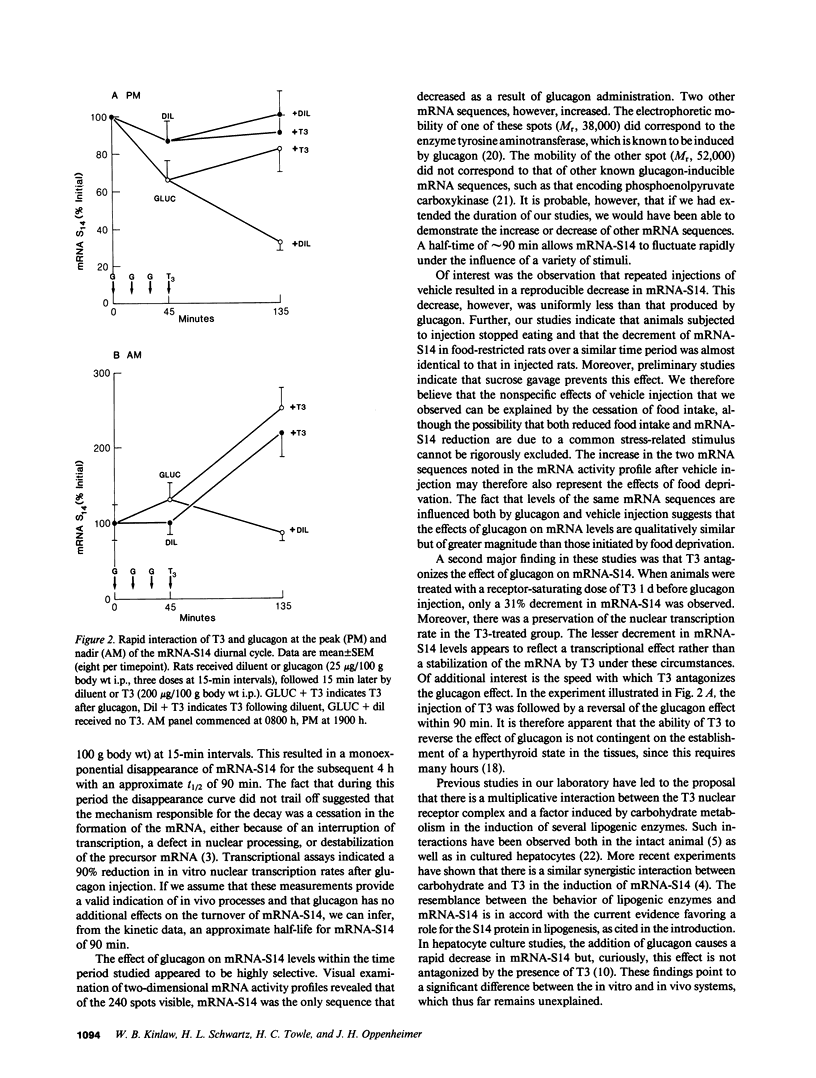
We have studied the effect of glucagon on the expression of a triiodothyronine (T3) and carbohydrate-inducible mRNA sequence (mRNA-S14) in rat liver that undergoes a threefold diurnal variation (peak, 2200 h; nadir, 0800 h). Glucagon injection into euthyroid rats (25 micrograms/100 g body wt i.p., three doses at 15-min intervals) during the nocturnal plateau of mRNA-S14 caused a monoexponential disappearance of this sequence (t1/2, 90 min) accompanied by a 90% reduction in the transcriptional rate in a nuclear run-off assay, indicative of a near total reduction of synthesis. This effect was markedly attenuated in rats treated with T3 (200 micrograms/100 g body wt i.p.) 24 h before glucagon injection. When T3 was given 15 min after glucagon, the glucagon-initiated decline in mRNA-S14 was reversed within 90 min, suggesting a rapid interaction between the two hormones in the evening. Curiously, administration of T3 alone at this hour did not affect a significant increase in mRNA-S14. At 0800 h, however, T3 caused the expected brisk induction of this sequence, whereas glucagon was without effect. In essence, glucagon affected mRNA-S14 synthesis only in the evening, while T3 increased levels of this sequence above the baseline only in the morning. T3, however, reversed the effect of prior glucagon injection at night. The observed alterations in hormonal responsivity could underly the diurnal variation of mRNA-S14 expression. Moreover, the data suggest the hypothesis that T3 may act on S14 gene expression by antagonizing factors that inhibit its transcription.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Hargrove J. L., Diesterhaft M., Noguchi T., Granner D. K. Identification of native tyrosine aminotransferase and an explanation for the multiple forms. J Biol Chem. 1980 Jan 10;255(1):71–78. [PubMed] [Google Scholar]
- Jump D. B., Narayan P., Towle H., Oppenheimer J. H. Rapid effects of triiodothyronine on hepatic gene expression. Hybridization analysis of tissue-specific triiodothyronine regulation of mRNAS14. J Biol Chem. 1984 Mar 10;259(5):2789–2797. [PubMed] [Google Scholar]
- Jump D. B., Oppenheimer J. H. High basal expression and 3,5,3'-triiodothyronine regulation of messenger ribonucleic acid S14 in lipogenic tissues. Endocrinology. 1985 Dec;117(6):2259–2266. doi: 10.1210/endo-117-6-2259. [DOI] [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw C. W., Towle H. C. Characterization of a thyroid hormone-responsive gene from rat. J Biol Chem. 1984 Jun 10;259(11):7253–7260. [PubMed] [Google Scholar]
- Mariash C. N., Jump D. B., Oppenheimer J. H. T3 stimulates the synthesis of a specific mRNA in primary hepatocyte culture. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1122–1129. doi: 10.1016/s0006-291x(84)80249-1. [DOI] [PubMed] [Google Scholar]
- Mariash C. N., Kaiser F. E., Schwartz H. L., Towle H. C., Oppenheimer J. H. Synergism of thyroid hormone and high carbohydrate diet in the induction of lipogenic enzymes in the rat. Mechanisms and implications. J Clin Invest. 1980 May;65(5):1126–1134. doi: 10.1172/JCI109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariash C. N., Oppenheimer J. H. Stimulation of malic enzyme formation in hepatocyte culture by metabolites: evidence favoring a nonglycolytic metabolite as the proximate induction signal. Metabolism. 1984 Jun;33(6):545–552. doi: 10.1016/0026-0495(84)90010-6. [DOI] [PubMed] [Google Scholar]
- Mariash C. N., Seelig S., Oppenheimer J. H. A rapid, inexpensive, quantitative technique for the analysis of two-dimensional electrophoretograms. Anal Biochem. 1982 Apr;121(2):388–394. doi: 10.1016/0003-2697(82)90498-5. [DOI] [PubMed] [Google Scholar]
- Mariash C. N., Seelig S., Schwartz H. L., Oppenheimer J. H. Rapid synergistic interaction between thyroid hormone and carbohydrate on mRNAS14 induction. J Biol Chem. 1986 Jul 25;261(21):9583–9586. [PubMed] [Google Scholar]
- Munnich A., Daegelen D., Besmond C., Marie J., Dreyfus J. C., Kahn A. Cell-free translation of messenger RNAs from human muscle biopsies: a miniaturized tool for investigation of neuromuscular diseases. Pediatr Res. 1982 May;16(5):335–339. doi: 10.1203/00006450-198205000-00001. [DOI] [PubMed] [Google Scholar]
- Narayan P., Liaw C. W., Towle H. C. Rapid induction of a specific nuclear mRNA precursor by thyroid hormone. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4687–4691. doi: 10.1073/pnas.81.15.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P., Towle H. C. Stabilization of a specific nuclear mRNA precursor by thyroid hormone. Mol Cell Biol. 1985 Oct;5(10):2642–2646. doi: 10.1128/mcb.5.10.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Seelig S., Jump D. B., Towle H. C., Liaw C., Mariash C. N., Schwartz H. L., Oppenheimer J. H. Paradoxical effects of cycloheximide on the ultra-rapid induction of two hepatic mRNA sequences by triiodothyronine (T3). Endocrinology. 1982 Feb;110(2):671–673. doi: 10.1210/endo-110-2-671. [DOI] [PubMed] [Google Scholar]
- Seelig S., Liaw C., Towle H. C., Oppenheimer J. H. Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4733–4737. doi: 10.1073/pnas.78.8.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui U. A., Goldflam T., Goodridge A. G. Nutritional and hormonal regulation of the translatable levels of malic enzyme and albumin mRNAs in avian liver cells in vivo and in culture. J Biol Chem. 1981 May 10;256(9):4544–4550. [PubMed] [Google Scholar]
- Srikant C. B., Freeman D., McCorkle K., Unger R. H. Binding and biologic activity of glucagon in liver cell membranes of chronically hyperglucagonemic rats. J Biol Chem. 1977 Nov 10;252(21):7434–7438. [PubMed] [Google Scholar]
- Tiedgen M., Seitz H. J. Dietary control of circadian variations in serum insulin, glucagon and hepatic cyclic AMP. J Nutr. 1980 May;110(5):876–882. doi: 10.1093/jn/110.5.876. [DOI] [PubMed] [Google Scholar]
- Winberry L. K., Morris S. M., Jr, Fisch J. E., Glynias M. J., Jenik R. A., Goodridge A. G. Molecular cloning of cDNA sequences for avian malic enzyme. Nutritional and hormonal regulation of malic enzyme mRNA levels in avian liver cells in vivo and in culture. J Biol Chem. 1983 Jan 25;258(2):1337–1342. [PubMed] [Google Scholar]


