Abstract
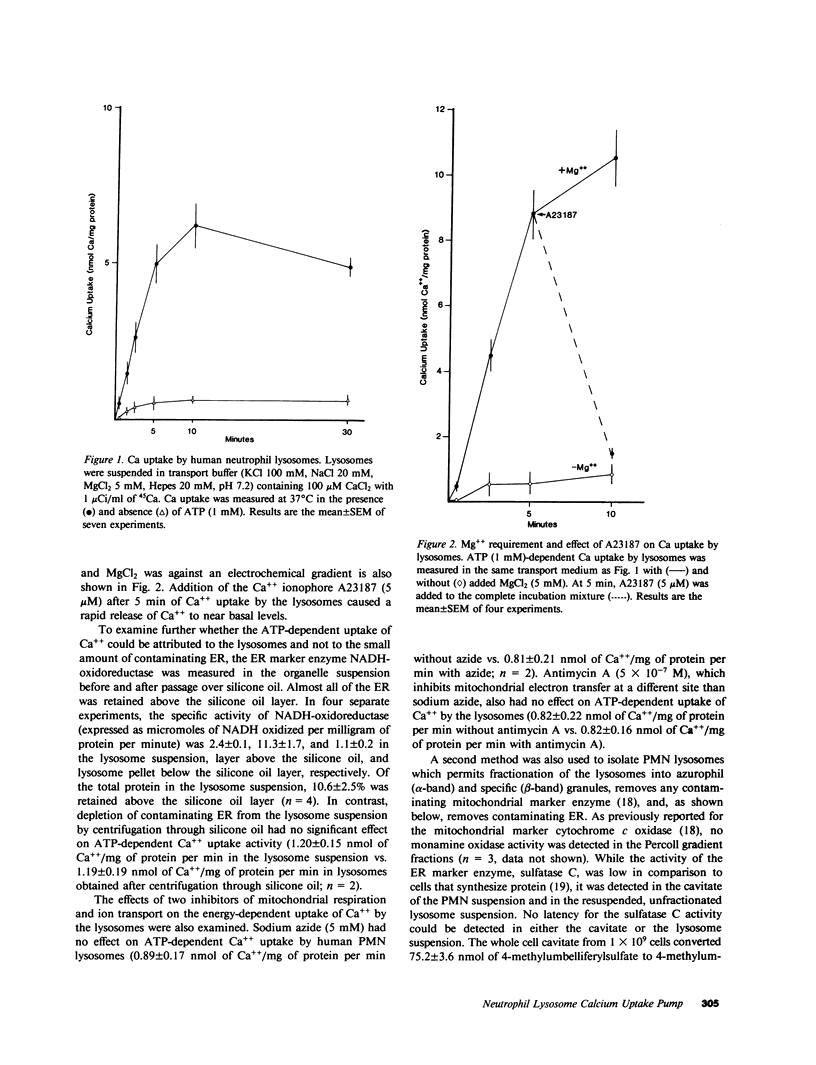
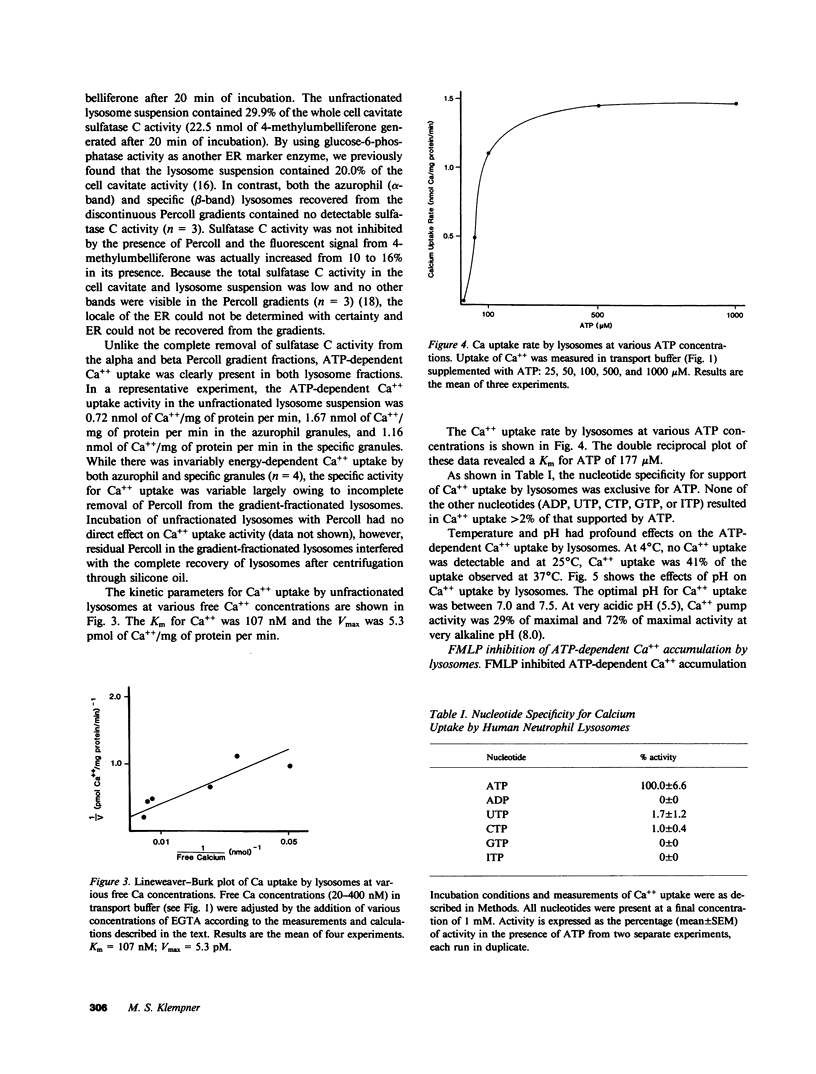
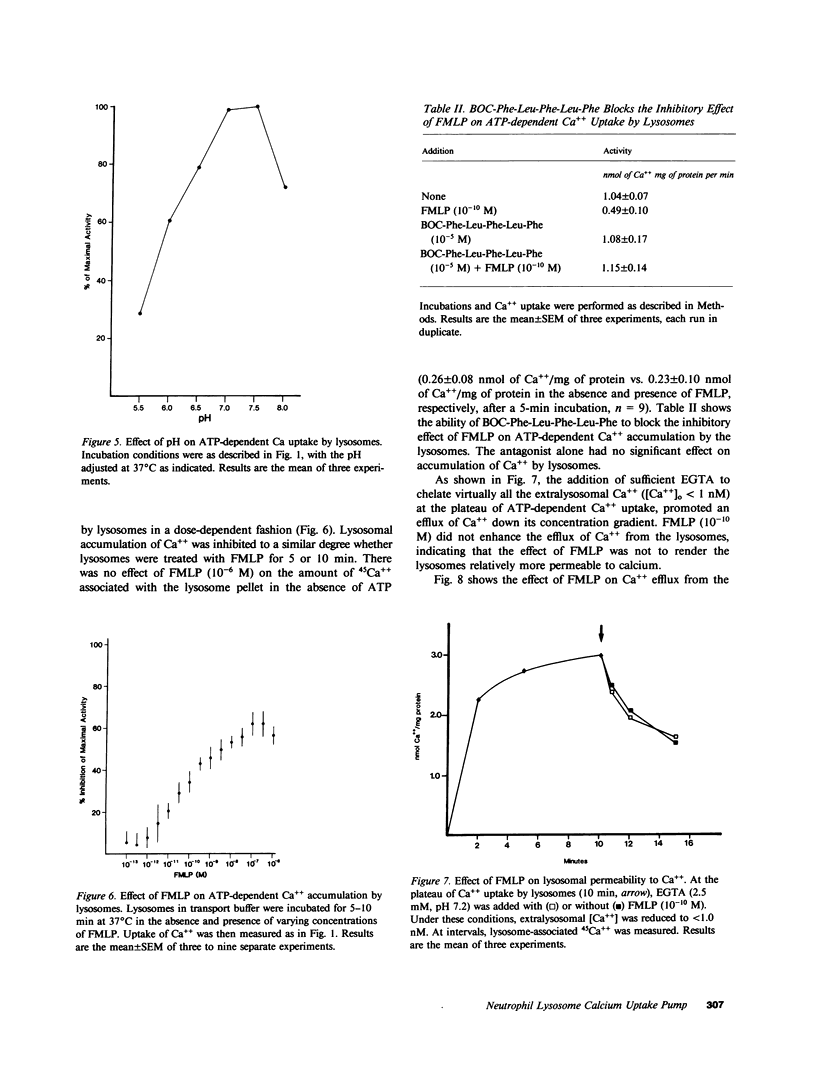
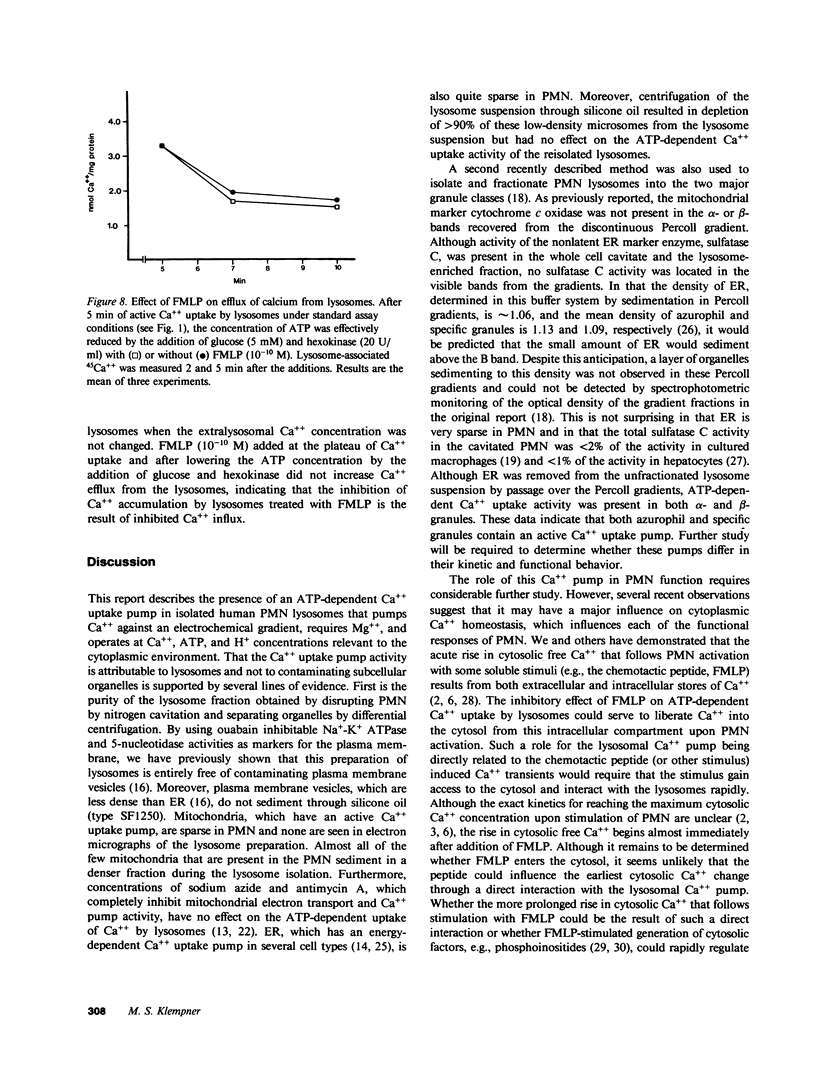
Regulation of the cytosolic free calcium concentration is important to neutrophil function. In these studies, an ATP-dependent calcium uptake pump has been identified in human neutrophil lysosomes. This energy-dependent Ca++ uptake pump has a high affinity for Ca++ (Michaelis constant [Km] Ca++ = 107 nM) and a maximum velocity (Vmax) of 5.3 pmol/mg of protein per min. ATP was the only nucleotide that supported Ca++ uptake by lysosomes. The Km for ATP was 177 microM. ATP-dependent Ca++ uptake by neutrophil lysosomes was temperature- and pH-sensitive with optimal Ca++ pump activity at 37 degrees C and pH 7.0-7.5. Mg++ was also essential for ATP-dependent Ca++ uptake by lysosomes. Azide and antimycin A had no effect on the energy-dependent uptake of Ca++ by neutrophil lysosomes. The chemotactic peptide formyl-methionyl-leucyl-phenylalanine inhibited ATP-dependent Ca++ accumulation by isolated lysosomes. Butoxycarbonyl-phenylalanine-leucine-phenylalanine-leucine-phenylalanine , a competitive antagonist of the chemotactic peptide, blocked this inhibitory effect. These studies demonstrate the presence of an ATP-dependent Ca++ uptake pump in human neutrophil lysosomes that functions at physiologic intracellular concentrations of Ca++, ATP, and H+ and may be important to regulating neutrophil function by modulating cytosolic Ca++.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Canonico P. G., Beaufay H., Nyssens-Jadin M. Analytical fractionation of mouse peritoneal macrophages: physical and biochemical properties of subcellular organelles from resident (unstimulated) and cultivated cells. J Reticuloendothel Soc. 1978 Aug;24(2):115–138. [PubMed] [Google Scholar]
- Carafoli E., Crompton M. The regulation of intracellular calcium by mitochondria. Ann N Y Acad Sci. 1978 Apr 28;307:269–284. doi: 10.1111/j.1749-6632.1978.tb41957.x. [DOI] [PubMed] [Google Scholar]
- Fletcher M. P., Gallin J. I. Human neutrophils contain an intracellular pool of putative receptors for the chemoattractant N-formyl-methionyl-leucyl-phenylalanine. Blood. 1983 Oct;62(4):792–799. [PubMed] [Google Scholar]
- Fletcher M. P., Seligmann B. E., Gallin J. I. Correlation of human neutrophil secretion, chemoattractant receptor mobilization, and enhanced functional capacity. J Immunol. 1982 Feb;128(2):941–948. [PubMed] [Google Scholar]
- Hirata M., Hamachi T., Hashimoto T., Suematsu E., Koga T. Ca2+ release in the endoplasmic reticulum of guinea pig peritoneal macrophages. J Biochem. 1983 Oct;94(4):1155–1163. doi: 10.1093/oxfordjournals.jbchem.a134460. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Mikkelsen R. B., Corfman D. H., André-Schwartz J. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 1980 Jul;86(1):21–28. doi: 10.1083/jcb.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner M. S., Styrt B. Clindamycin uptake by human neutrophils. J Infect Dis. 1981 Nov;144(5):472–479. doi: 10.1093/infdis/144.5.472. [DOI] [PubMed] [Google Scholar]
- Lagast H., Lew P. D., Waldvogel F. A. Adenosine triphosphate-dependent calcium pump in the plasma membrane of guinea pig and human neutrophils. J Clin Invest. 1984 Jan;73(1):107–115. doi: 10.1172/JCI111180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagast H., Pozzan T., Waldvogel F. A., Lew P. D. Phorbol myristate acetate stimulates ATP-dependent calcium transport by the plasma membrane of neutrophils. J Clin Invest. 1984 Mar;73(3):878–883. doi: 10.1172/JCI111284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L., Reynafarje B., Vercesi A., Tew W. P. Transport and accumulation of calcium in mitochondria. Ann N Y Acad Sci. 1978 Apr 28;307:160–176. doi: 10.1111/j.1749-6632.1978.tb41941.x. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Stossel T. P. Calcium transport by macrophage plasma membranes. J Biol Chem. 1980 Jun 25;255(12):5841–5846. [PubMed] [Google Scholar]
- Lew P. D., Wollheim C., Seger R. A., Pozzan T. Cytosolic free calcium changes induced by chemotactic peptide in neutrophils from patients with chronic granulomatous disease. Blood. 1984 Jan;63(1):231–233. [PubMed] [Google Scholar]
- Moore L., Pastan I. Energy-dependent calcium uptake by fibroblast microsomes. Ann N Y Acad Sci. 1978 Apr 28;307:177–194. doi: 10.1111/j.1749-6632.1978.tb41942.x. [DOI] [PubMed] [Google Scholar]
- Ochs D. L., Reed P. W. ATP-dependent calcium transport in plasma membrane vesicles from neutrophil leukocytes. J Biol Chem. 1983 Aug 25;258(16):10116–10122. [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The roles of extracellular and intracellular calcium in lysosomal enzyme release and superoxide anion generation by human neutrophils. Biochim Biophys Acta. 1981 Nov 5;677(3-4):512–520. doi: 10.1016/0304-4165(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Southwick F. S., Stossel T. P. Contractile proteins in leukocyte function. Semin Hematol. 1983 Oct;20(4):305–321. [PubMed] [Google Scholar]
- Thayer W. S., Rubin E. Antimycin inhibition as a probe of mitochondrial function in isolated rat hepatocytes. Effects of chronic ethanol consumption. Biochim Biophys Acta. 1982 Dec 30;721(4):328–335. doi: 10.1016/0167-4889(82)90086-6. [DOI] [PubMed] [Google Scholar]
- Vincenzi F. F., Adunyah E. S., Niggli V., Carafoli E. Purified red blood cell Ca2+-pump ATPase: evidence for direct inhibition by presumed anti-calmodulin drugs in the absence of calmodulin. Cell Calcium. 1982 Dec;3(6):545–559. doi: 10.1016/0143-4160(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Sha'afi R. I. Calcium transport in inside-out membrane vesicles prepared from rabbit neutrophils. J Biol Chem. 1983 Apr 10;258(7):4153–4158. [PubMed] [Google Scholar]
- Volpi M., Yassin R., Naccache P. H., Sha'afi R. I. Chemotactic factor causes rapid decreases in phosphatidylinositol,4,5-bisphosphate and phosphatidylinositol 4-monophosphate in rabbit neutrophils. Biochem Biophys Res Commun. 1983 May 16;112(3):957–964. doi: 10.1016/0006-291x(83)91711-4. [DOI] [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Molski T. F., Borgeat P., Sha'afi R. I. Direct demonstration of increased intracellular concentration of free calcium in rabbit and human neutrophils following stimulation by chemotactic factor. Biochem Biophys Res Commun. 1983 May 31;113(1):44–50. doi: 10.1016/0006-291x(83)90429-1. [DOI] [PubMed] [Google Scholar]
- Yano K., Nakashima S., Nozawa Y. Coupling of polyphosphoinositide breakdown with calcium efflux in formyl-methionyl-leucyl-phenylalanine-stimulated rabbit neutrophils. FEBS Lett. 1983 Sep 19;161(2):296–300. doi: 10.1016/0014-5793(83)81028-x. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]


