Abstract
α2-adrenoceptors are widely distributed throughout the central nervous system (CNS) and the systemic administration of α2-agonists such as dexmedetomidine produces clinically useful, centrally-mediated sedation and analgesia; however, these same actions also limit the utility of these agents (ie unwanted sedative actions). Despite a wealth of data on cellular and synaptic actions of α2-agonists in vitro, it is not known which neuronal circuits are modulated in vivo to produce the analgesic effect. To address this issue, we made in vivo recordings of membrane currents and synaptic activities in superficial spinal dorsal horn neurons and examined their responses to systemic dexmedetomidine. We found that dexmedetomidine at doses that produce analgesia (<10 μg/kg) enhanced inhibitory postsynaptic transmission within the superficial dorsal horn without altering excitatory synaptic transmission or evoking direct postsynaptic membrane currents. In contrast, higher doses of dexmedetomidine (>10 μg/kg) induced outward currents by a direct postsynaptic action. The dexmedetomidine-mediated inhibitory postsynaptic current (IPSC) facilitation was not mimicked by spinal application of dexmedetomidine and was absent in spinalized rats, suggesting it acts at a supraspinal site. Further it was inhibited by spinal application of the α1-antagonist prazosin. In the brain stem, low doses of systemic dexmedetomidine produced an excitation of locus coeruleus neurons. These results suggest that systemic α2-adrenoceptor stimulation may facilitate inhibitory synaptic responses in the superficial dorsal horn to produce analgesia mediated by activation of the pontospinal noradrenergic inhibitory system. This novel mechanism may provide new targets for intervention perhaps allowing analgesic actions to be dissociated from excessive sedation.
1. Introduction
Clinically α2-adrenoceptor agonists such as dexmedetomidine are widely used as sedative agents, as adjuncts to anesthesia and have been noted to produce analgesic effects [9; 13; 38]. Dexmedetomidine is a potent analgesic but the accompanying sedation (and hypotension) produced by systemic administration has limited the widespread deployment of α2-agonists as analgesics and prompted investigations to separate these effects. It has been suggested that the sedative action of dexmedetomidine is primarily mediated by inhibition of locus coeruleus (LC) neurons, which has an important role in attention and arousal [2; 4; 12; 15]. Whereas there is evidence suggesting that the spinal cord is the principal site for its analgesic action [35; 61], and previous behavioral studies have shown that intrathecal or epidural dexmedetomidine is antinociceptive [3; 18]. However, there is conflicting evidence suggesting that after systemic administration it may act to produce analgesia via an action at the LC [26].
In addition to the LC and spinal cord, α2-adrenoceptors are widely distributed throughout the central nervous system (CNS) and mediate responses to the release of endogenous central catecholamines [24; 43]. Activation of postsynaptic α2-adrenoceptors produces hyperpolarization by the activation of G protein-coupled inwardly-rectifying potassium channels via Gi/o-proteins. Presynaptic α2-adrenoceptors reduce neurotransmitter release by inhibiting calcium influx (see review [52]). In the noradrenergic neurons of the LC, α2-adrenoceptors act as autoreceptors to reduce local noradrenaline release [33] and also induce postsynaptic hyperpolarization [2; 12; 15].
Within spinal nociceptive circuits, the substantia gelatinosa (SG, lamina II) neurons in the superficial dorsal horn play an important role in the transmission and modulation of nociceptive information [11; 20]. The SG receives nociceptive information via glutamatergic synapses from peripheral Aδ- and C- afferent fibers [22; 42; 44; 47; 63; 66]. SG neurons also receive abundant inhibitory synaptic inputs from spinal GABAergic and glycinergic interneurons [7; 41; 48; 68] and receive a dense innervation from a descending inhibitory system including noradrenergic fibers from the LC [8; 30; 50; 55]. A number of in vitro studies have shown spinal α2-agonists to produce both post-synaptic hyperpolarization [49] and presynaptic inhibition of excitatory transmission [37]. A recent electrophysiological study has specifically shown dexmedetomidine to have a direct inhibitory action on SG neurons in vitro [31]. It is likely that these mechanisms mediate the analgesic effects of spinally administered α2-agonists, but it is not known whether the systemic administration of dexmedetomidine acts to modulate spinal nociceptive processing in a similar manner.
We have developed an in vivo patch-clamp recording technique from SG neurons in allowing the detailed analysis of synaptic responses and changes in intrinsic membrane properties [19; 22]. We used this approach to test the hypothesis that systemic dexmedetomidine, at analgesic doses, acts to inhibit SG neurons in vivo by one of the previously reported mechanisms [37; 49]. We found that low-dose dexmedetomidine (at doses below that shown to have sedative actions) dramatically enhanced spinal inhibitory transmission (without effects on excitatory transmission or intrinsic membrane conductances) by activation of the pontospinal descending noradrenergic circuit.
2. Materials and methods
2.1 Animals
Ninety male Sprague-Dawley rats aged 5-9 weeks, weighing 150-350 g were used in this study. The animals were purchased from Japan SLC (Hamamatsu, Japan) and housed in a temperature-controlled room (21 ± 1°C) with a 12 hour light/dark cycle, and given free access to food and water. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the National Institutes of Natural Sciences, and were performed in accordance with the institutional guidelines for animal experiments and were consistent with the ethical guidelines of the International Association for the Study of Pain. All efforts were made to reduce the number of animals for this study. At the end of the experiment, the animals were killed with supplemental administration of urethane (2-4 g/kg, i.p.).
2.2 Spinal nerve ligation model
Rats aged 5 weeks were used for the spinal nerve ligation model. Under sevoflurane anesthesia (3-5 %in O2, delivered by mask) the right L5 and L6 spinal nerves were isolated and ligated tightly with 6-0 silk suture, according to the method of Chung [39]. One week after the surgery, the withdrawal threshold of the ipsilateral hind paw was assessed with von Frey filaments. Animals whose withdrawal threshold was below 8 g (6 weeks old) were used for in vivo patch-clamp recordings.
2.3 Behavioral tests
Behavioral testing was carried out in a quiet room away from the colony room in daylight and at standard temperature (24 ± 1°C). Thirteen rats aged 6 weeks were included in this protocol. The rats were allowed to acclimatise to the test location for ~1 hour before the experiment. Rats were put onto a perforated metal platform, and mechanical stimuli were delivered to the plantar surface of the hind paw using the Dynamic Plantar Aesthesiometer (37450, Ugo Basile, Comerio, Italy) positioned beneath the platform. The equipment raises a straight metal filament of diameter of 0.5 mm until it touches the plantar surface of the hindpaw and exerts an increasing upwards force (from 1 to 50 g over 20 s) until the paw is withdrawn or the preset cut-off is reached (50 g). Dexmedetomidine was administered intraperitoneally sequentially from low to high dose in the same rat. Twenty minutes after the administration of each dose, the mechanical withdrawal threshold was measured for the right paw from the average of five Aesthesiometer trials.
Six rats aged 6 weeks were used for sedation assessment. The observation chambers for sedation assessment were 20×20×14cm clear plastic cage with mesh floor. The rats were habituated to the chamber for ~1 hour before the experiment. All rats were administered 0.01, 0.1, 1, 10 and 30 μg/kg of dexmedetomidine intraperitoneally in a sequential manner. The sedation assessments were performed 20 minutes after each drug administration. We adopted the sedation rating scale of Chuck et al. [14]. The ratings were as follows: 5-awake, active: engaged in locomotion, rearing, head movements or grooming; 4-awake, inactive: eyes fully open, head up, little to no locomotion, rearing or grooming, normal posture; 3-mild sedation: eyes partly closed, head somewhat down, impaired locomotion including abnormal posture, use only some limbs, dragging and stumbling; 2-moderate sedation: head mostly or completely down, eyes partly closed, flattened posture, no spontaneous movement; 1-heavy sedation: eyes mostly closed, loss of righting reflex; 0-asleep: eyes fully closed, body relaxed, asleep.
2.4 In vivo patch-clamp recording from SG neurons
The methods used for the in vivo patch-clamp recording from the SG were similar to those described previously [19; 21; 58]. Briefly, the rats (n = 66) were anesthetized with urethane (1.2-1.5 g/kg, i.p.). Thoracolumbar laminectomy was performed at the level of T12 to L2 to expose the lumbar enlargement of the spinal cord. In the case of spinalized rats (3 rats), an additional laminectomy was made at the cervical level to allow right-sided cord hemisection to interrupt descending inhibitory pathways ipsilateral to the recording site. The rat was placed in a stereotaxic apparatus (Model ST-7, Narishige, Tokyo, Japan). After the dura mater was opened, the pia-arachnoid membrane was cut to make a window to allow the patch electrode to enter into the SG. The surface of the spinal cord was irrigated with 95 % O2 - 5 % CO2 equilibrated Krebs solution (in mM: 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 11 glucose, and 25 NaHCO3) at a flow rate of 10-15 ml/min at 38 ± 1 °C. Patch electrodes were fabricated from thin-walled borosilicate glass capillaries using a puller (p-97, Sutter Instrument, Novato, CA), and had resistances of 8-15 MΩ when filled with either of potassium-based (in mM: 135 K-gluconate, 5 KCl, 0.5 CaCl2, 2 MgCl2, 5 EGTA, 5 Mg-ATP, 5 HEPES, pH 7.2 adjusted with KOH) or cesium-based (in mM: 110 Cs2SO4, 5 tetraethylammonium, 0.5 CaCl2, 2 MgCl2, 5 EGTA, 5 Mg-ATP, 5 HEPES, pH 7.2 adjusted with CsOH) intracellular solutions. The potassium- and cesium- solutions were used for recordings of outward currents and excitatory postsynaptic currents (EPSCs) at a holding potential of −70 mV and inhibitory postsynaptic currents (IPSCs) at 0 mV, respectively.
The patch electrode was advanced into the spinal cord using a micromanipulator (Model MHW-4, Narishige). Blind whole-cell recordings [67] were obtained from SG neurons at a depth of 30-200 μm from the surface [22]. The recorded currents were amplified (Axopatch 200B, Molecular Devices, Sunnyvale, CA) and digitized (Digidata 1321A, Molecular Devices) for storage/analysis on a personal computer using a data acquisition program (Clampex version 10.3, Molecular Devices). The frequencies and amplitudes of EPSCs/IPSCs were analyzed with MiniAnalysis software (Synaptosoft, Fort Lee, NJ). The total area of IPSCs over a 10 second period (before and in the presence of α2-agonist) was measured with Clampfit software (version 10.3, Molecular Devices) reflecting the ongoing synaptic charge transfer (pC).
2.5 Extracellular recording from the LC
Eight rats aged 5-8 weeks were included in this protocol. Conventional extracellular and in vivo cell-attached patch-clamp recordings were obtained from LC neurons as described previously [51; 59]. Under urethane anesthesia (1.2-1.5 g/kg, i.p.), rats were mechanically ventilated after tracheostomy and bilateral thoracotomy was performed to reduce the respiratory movement. The head was fixed in a stereotaxic apparatus (Model SR-5R, Narishige). The floor of the fourth ventricle was exposed through a posterior occipital craniotomy using gentle suction aspiration to remove the central portion of the cerebellum. The surface of the exposed brainstem was irrigated with Krebs solution equilibrated with 95 % O2 -5 % CO2 at 38 ± 0.5°C at a flow rate of 5-10 ml/min. A tungsten electrode (impedance, 1 MΩ, A-M systems, Sequim, WA) electrode was placed into the LC and action potentials in LC neurons were extracellularly recorded with an AC differential amplifier (DAM 80, World Precision Instruments, Sarasota, FL). Firing rate of LC neurons was analyzed with Offline Sorter software (version 3, Plexon, Dallas, TX). In some experiments cell-attached patch-clamp recordings were made from LC neurons allowing action potential discharge to be monitored [59]. LC neurons were identified on the basis of their characteristic spontaneous firing and responses elicited by pinch stimulation applied to the contralateral hind limb [59]. The spontaneous firing rate of the LC neurons in the present study was higher than that (2-3 Hz) reported previously [50]. This is probably due to our experimental conditions for example the laminectomy and cerebellectomy may tend to increase the firing frequency. The value is similar to that of LC neurons reported by in vivo patch-clamp recordings in a similar preparation [59].
2.6 Application of drugs
Dexmedetomidine diluted in saline was intraperitoneally injected 20 min before behavioral tests, and administered via a left femoral vein catheter during in vivo patch-clamp and extracellular recordings. For spinal or LC application drugs were diluted in Krebs solution and superfused onto the surface of the brainstem/cord. We arbitrarily defined neurons as being sensitive to a particular drug when the frequency, amplitude or area of the synaptic responses was altered by more than 20 % of control. The actions of systemic dexmedetomidine on IPSCs were examined in SG neurons of rats aged 5-9 weeks. We tested for an effect of age on the action of dexmedetomidine by comparing results from rats of 5-6 weeks with those aged 7-9 weeks and found no difference between the groups. The drugs used were dexmedetomidine (Waterstone Technology, Carmel, IN), L-(−)- norepinephrine (+)- bitartrate salt monohydrate, clonidine, tetrodotoxin, prazosin and yohimbine (all from Sigma, St. Louis, MO).
2.7 Statistical analysis
All numerical data are shown as mean ± SEM. Statistical significance was determined as p < 0.05 using the paired Student’s t-test, one-way ANOVA with Dunnett’s post hoc test (for withdrawal threshold data), the Kruskal-Wallis test followed by the Stell-Dwass test (for sedative assessment data) or Kolmogorov-Smirnov test (cumulative distributions of IPSCs).
3. Results
3.1 Analgesic effects of systemic dexmedetomidine on mechanical nociception
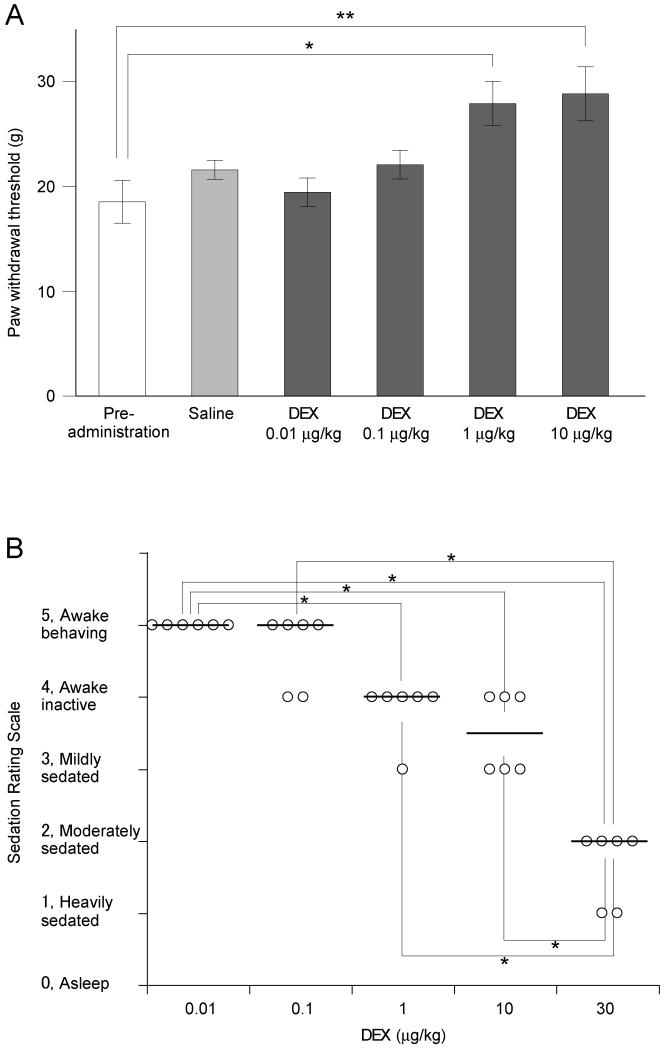
We undertook a behavioral analysis to find the systemic dose range over which dexmedetomidine has analgesic effects. We used a dynamic aesthesiometer (Ugo Basile, Italy) to evaluate the effect of dexmedetomidine (ascending doses 0.01-10 μg/kg) on mechanical withdrawal threshold. The mechanical baseline withdrawal threshold was 18.5 ± 2.0 g (n = 13). Dexmedetomidine dose-dependently increased the mechanical withdrawal thresholds at doses from 1 μg/kg (27.9 ± 2.1 g, p < 0.01; Fig. 1A). In contrast intraperitoneal injection of saline did not change the threshold (control, 20.6 ± 0.8 g; saline, 21.6 ± 0.9 g; n = 4; p > 0.05). Dexmedetomidine (1-10 μg/kg) had demonstrable analgesic actions on mechanical noxious withdrawal response at doses well below the previously reported sedative range [10; 16; 54].
Figure 1. Dose-dependent anti-nociceptive and sedative action of systemic dexmedetomidine.
A) Paw withdrawal thresholds to mechanical stimuli were measured in conscious animals using the Dynamic Plantar Aesthesiometer 20 min after intraperitoneal administration of dexmedetomidine (DEX, 0.01 to 10 μg/kg, n = 13). Dexmedetomidine significantly increased the withdrawal threshold at doses of 1 μg/kg (*p < 0.05) and 10 μg/kg (**p < 0.01) compared control prior to drug administration (n = 13, one-way ANOVA and Dunnett’s post hoc test).
B) The sedation rating scores were assessed 20 minutes after intraperitoneal dexmedetomidine (DEX, 0.01 to 10 μg/kg, n = 6). Median scores were significantly decreased at 1 μg/kg (*p < 0.05) and 10 μg/kg (**p < 0.01) compared with pre-administration control (Kruskal-Wallis test followed by the Stell-Dwass test) although most of the animals were considered to be still awake or only mildly sedated in this dose range. Only those animals receiving 30 μg/kg showed evidence of moderate to heavy sedation which was a significantly greater degree of sedation than that produced by either 1 or 10 μg/kg (p < 0.05).
3.2 Sedative effects of systemic dexmedetomidine
We performed a sedation assessment across a range of systemic dexmedetomidine doses. The sedative rating score (see section 2.4) before the administration of dexmedetomidine was 5 in all rats (n = 6). Intraperitoneal dexmedetomidine (ascending doses 0.01-30 μg/kg) dose-dependently decreased the sedation score showing statistical significance at doses from 1 μg/kg (Fig. 1B). The median sedation scores were 5 at 0.01 and 0.1 μg/kg – awake and active; 4 at 1 μg/kg– awake but inactive (p < 0.05); 3.5 at 10 μg/kg - awake but inactive to mild sedation (p < 0.01) and 2 at 30 μg/kg moderate to heavy sedation (p < 0.01). Thus a dose of 30 μg/kg of systemic dexmedetomidine was needed to produce moderate to heavy sedation - equivalent to clinical sedative levels. These results were similar to those reported for the effect of dexmedetomidine on EEG by Bol et al.[10].
3.3 Cellular mechanism of action of systemic dexmedetomidine on SG neurons
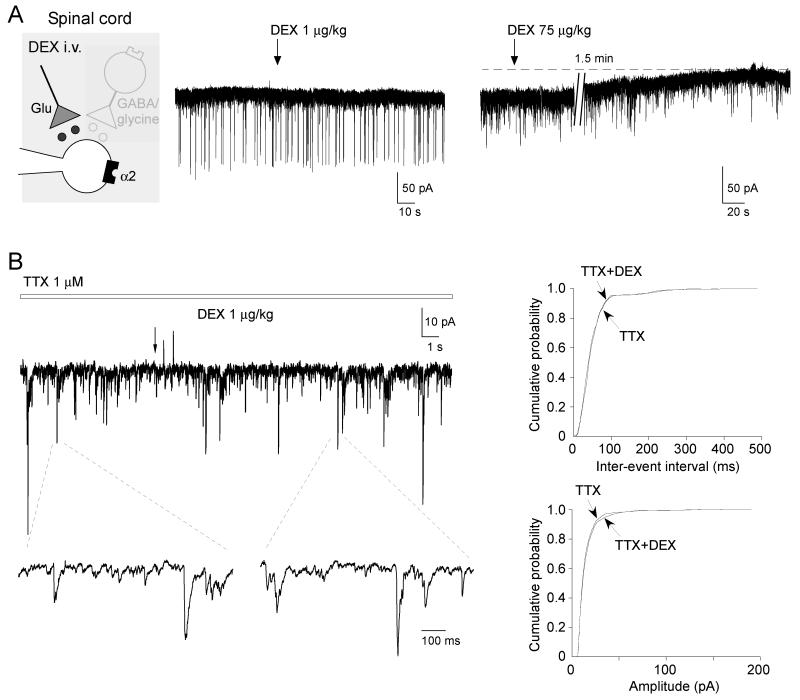
Ishii et al. showed that bath application of dexmedetomidine activated potassium conductances (EC50 of 0.62 μM) to directly inhibit SG neurons in acute spinal cord slices [31]. We therefore tested whether low dose systemic dexmedetomidine (1-10 μg/kg) elicited outward currents in SG neurons voltage clamped (Vh −70 mV) in whole cell patch clamp configuration in vivo using naive and Chung model rats. All neurons studied had membrane potentials more negative than −50 mV. The average membrane potential and the input membrane resistance were −62 ± 1.2 mV (n = 53) and 384.8 ± 28.3 MΩ (n = 53), respectively for neurons recorded with the potassium-based intracellular solution. Administration of low dose systemic dexmedetomidine did not induce any significant persistent outward currents in vivo (as would be expected to follow post-synaptic α2-adrenoceptor activation, none detectable at 1 μg/kg, n = 7; 7.0 ± 2.6 pA at 10 μg/kg, n = 7). However it was noted that higher doses of systemic dexmedetomidine (>30μg/kg) were indeed capable of inducing long lasting outward currents, 23.0 ± 6.2 pA (n = 3), (Fig. 2A, 3C). In Chung model rats, administration of dexmedetomidine (1 μg/kg) did not induce detectable outward currents in any neuron tested (n = 7).
Figure 2. Effect of systemic dexmedetomidine on SG neuron excitability.
A) To look for α2-adrenoceptor mediated activation of potassium currents, we voltage clamped SG neurons (holding potential of −70mV with a potassium-based intracellular solution). The application of low doses of dexmedetomidine (1 μg/kg) did not elicit any detectable outward currents (left trace). In contrast, much higher doses of dexmedetomidine (30-75 μg/kg) were required to produce any sign of an outward current (right trace).
B) In the presence of tetrodotoxin (TTX), systemic administration of dexmedetomidine (1 μg/kg) did not enhance miniature EPSCs. The traces below are shown on an expanded time base to demonstrate the resolution of individual miniature EPSCs. The cumulative probability plots for the inter-event interval and amplitude of miniature EPSCs (from the trace in B), showing dexmedetomidine has no effect on either the frequency or amplitude (p > 0.05, Kolmogorov-Smirnov test).
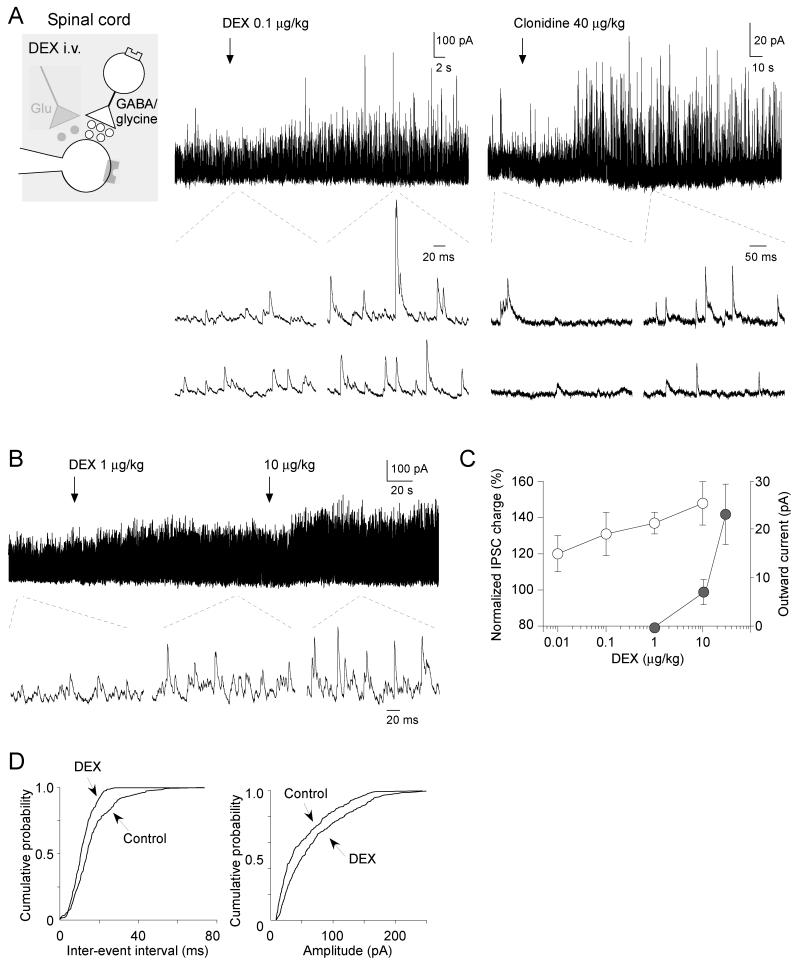
Figure 3. Systemic dexmedetomidine dose-dependently enhances spontaneous IPSCs in the SG.
A) Systemic dexmedetomidine (left trace, 0.1 μg/kg) and clonidine (right trace, 40 μg/kg) elicited barrages of spontaneous IPSCs (holding potential of 0 mV with a cesium-based based solution). The traces below are shown on an expanded time base to demonstrate the individual IPSCs.
B) Dexmedetomidine dose-dependently elicits barrages of spontaneous IPSCs.
C) Summary plot showing the enhancement of IPSCs (open circles) at dexmedetomidine doses (<10 μg/kg) that are lower than those required to induce outward currents (closed circles).
D) The cumulative probability plots for the inter-event interval and amplitude of spontaneous IPSCs (from the left trace in A). Dexmedetomidine at a dose of 0.1 μg/kg significantly increased the frequency (p < 0.01) and amplitude (p < 0.01) of spontaneous IPSCs (Kolmogorov-Smirnov test).
Given the known inhibition of excitatory synaptic transmission by α2-agonists [37], we tested for an effect of systemic dexmedetomidine on spontaneous EPSCs in SG neurons. Dexmedetomidine (1-30 μg/kg) did not change the frequency (control, 23.3 ± 5.0 Hz versus Dex., 23.2 ± 5.0 Hz; p > 0.05, n = 7) or amplitude (control, 15.5 ± 3.1 pA versus Dex., 14.8 ± 2.8 pA; p > 0.05, n = 7) of spontaneous EPSCs. In the presence of tetrodotoxin (TTX), dexmedetomidine (1 μg/kg) did not change the frequency (control 6.0 ± 2.7 Hz versus + Dex., 5.9 ± 2.7 Hz; p > 0.05, n = 6) or amplitude (control, 14.2 ± 1.9 Hz versus + Dex., 14.0 ± 1.9 Hz; p > 0.05, n = 6) of miniature EPSCs (Fig. 2B).
Although there was no change in spontaneous and miniature EPSCs, we noted a clear and dramatic change in inhibitory synaptic transmission to SG neurons. At a holding potential of 0 mV, all SG neurons tested exhibited spontaneous IPSCs. There was a resting tone of inhibition with average frequency 48.5 ± 3.5 Hz (n = 69) and amplitude 30.9 ± 1.9 pA (n = 69). These values were similar to those reported in our previous in vivo patch clamp study [36]. Dexmedetomidine elicited a barrage of spontaneous IPSCs at doses as low as 0.1 μg/kg (shown in Fig. 3A). The enhancement of IPSCs by dexmedetomidine was detected in most SG neurons tested (19 of 21; Fig. 3A, B, C). Dexmedetomidine increased the inhibitory synaptic charge transfer (measured over a 10 second period) by 121 ± 9 % (n = 4) at 0.01 μg/kg; 134 ± 10 % (n = 9) at 0.1 μg/kg; 138 ± 5 % (n = 13) at 1 μg/kg and by 148 ± 12 % (n = 4) at 10 μg/kg compared to controls (316.6 ± 38.4 pC, n = 19) (Fig. 3C). Dexmedetomidine shifted the cumulative event distributions of spontaneous IPSCs to shorten the inter-event interval and increase their amplitude (Fig. 3D). Systemic administration of another α2-agonist clonidine (40 μg/kg) produced a similar effect with increased spontaneous IPSCs (Fig. 3A, right).
3.4 Site of action of low dose dexmedetomidine on IPSCs
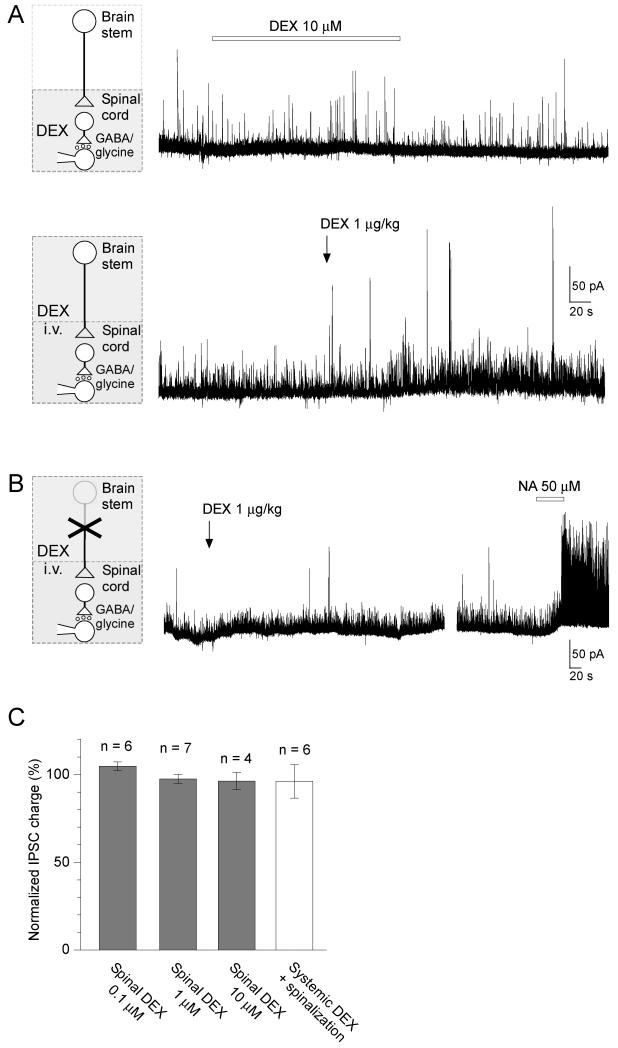
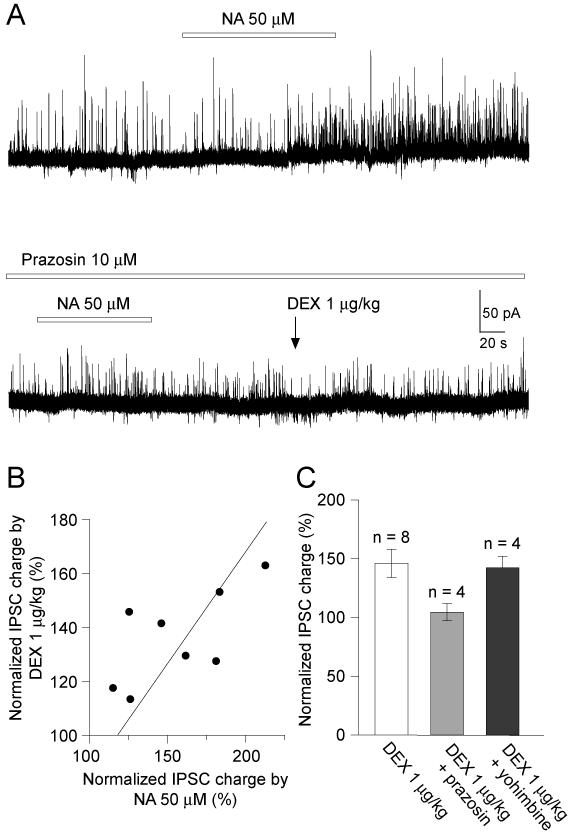
We next examined whether the enhancement of IPSCs by dexmedetomidine is mediated by a direct action at a spinal level. Dexmedetomidine (0.1-10 μM) applied by superfusion to the dorsal surface of the spinal cord [21] did not affect the spontaneous IPSCs (synaptic charge, 104.8 ± 2.4 % of control, n = 6, p > 0.05, at 0.1 μM; 97.5 ± 2.6 % of control, n = 7, p > 0.05, at 1 μM; 96.3 ± 4.9 % of control, n = 4, p > 0.05, at 10 μM) (Fig. 4A, C). In spinalised rats (cord hemisected ipsilaterally at cervical level) systemic dexmedetomidine (1 μg/kg) did not alter the spontaneous IPSCs (synaptic charge, 96.1 ± 9.4 %, n = 6, p > 0.05; Fig. 4B, C). In contrast, the direct application of noradrenaline (50 μM) to the surface of the spinal cord in spinalized rats provoked a striking barrage of IPSCs (Fig. 4B). These results suggest that low dose systemic dexmedetomidine facilitates IPSCs in SG neurons at a supraspinal level via a descending modulatory system.
Figure 4. Dexmedetomidine does not enhance IPSCs by a direct spinal action.
A) Spinal superfusion of dexmedetomidine (10 μM) had no effect on spontaneous IPSCs. In the same neuron, subsequent systemic application of dexmedetomidine (1 μg/kg) enhanced IPSCs.
B) Recordings from SG neurons in rats spinalized at the cervical level showed that systemic application of dexmedetomidine (1 μg/kg) no longer changed IPSC frequency or amplitude. However, spinal application of noradrenaline (NA, 50 μM) was able to dramatically facilitate IPSCs in the same neuron.
C) Summary showing effects of direct spinal superfusion of dexmedetomidine (0.1 – 10 μM), and systemic dexmedetomidine application in spinalized rats on normalized synaptic charge of spontaneous IPSCs.
3.5 Systemic dexmedetomidine engages the descending noradrenergic system to facilitate spontaneous IPSCs
Several previous in vitro patch-clamp studies have shown noradrenaline to facilitate spontaneous IPSCs in SG neurons in spinal cord slices – an action mediated through excitatory α1-adrenoceptors on inhibitory interneurons [5; 6; 23; 25]. On this basis we hypothesized that systemic dexmedetomidine may be acting via the descending noradrenergic system by disinhibiting LC neurons. Direct superfusion of noradrenaline (50 μM) to the surface of the spinal cord increased spontaneous IPSCs in 40 out of 48 SG neurons tested (Fig. 5A.; synaptic charge transfer increased to 219.8 ± 19.2 % of control). In 8 of 9 noradrenaline-sensitive cells, systemic dexmedetomidine (1 μg/kg) also enhanced spontaneous IPSCs (synaptic charge transfer increased to 157.2 ± 8.0 % of control). In each recorded neuron there was a strong correlation between the degree of IPSC facilitation seen with spinal noradrenaline and with systemic dexmedetomidine (n = 8, R2 = 0.96; Fig. 5B). Also consistent with this hypothesis was the observation that prazosin (an α1-antagonist, 10 μM) applied to the spinal cord blocked the facilitatory action of spinal Noradrenaline on spontaneous IPSCs (n = 4; Fig. 5A). Similarly the systemic dexmedetomidine facilitation of IPSCs was significantly suppressed by concurrent spinal application of prazosin (Dex., 323.3 ± 85.4 pC versus Dex. + prazosin, 226.3 ± 50.4 pC, n = 4, p < 0.05) (Fig. 5C). Spontaneous IPSCs were weakly but not significantly decreased by spinal superfusion of prazosin (10 μM) (synaptic charge, 90.9 ± 6.1 % of control, n = 6, p > 0.05). On the other hand, the facilitatory action of systemic dexmedetomidine (1 μg/kg) on spontaneous IPSCs was not antagonized by spinal superfusion of yohimbine (4 μM), an α2 antagonist (Fig. 5C, synaptic charge increased by Dex to 142.4 ± 9.9 % of control, n = 4, p = 0.06). The spontaneous IPSC were not affected by spinal application of yohimbine (synaptic charge, 102.5 ± 13.5 % of control, n = 4, p > 0.05).
Figure 5. Spinal noradrenaline and systemic dexmedetomidine facilitate IPSCs in the same SG neurons.
A) In this neuron the facilitation of IPSCs by local superfusion of noradrenaline (NA, 50 μM, shown in upper trace) and systemic dexmedetomidine is blocked by spinal application of the α1-adrenoceptor antagonist prazosin (10 μM, lower trace). Data in A were obtained from the same neurons shown in Fig. 4A.
B) There was a strong correlation between the facilitatory actions of spinal noradrenaline and systemic dexmedetomidine on spontaneous IPSCs evoked in the same SG neurons (linear regression R2 = 0.96).
C) Summary chart showing the facilitatory action of systemic dexmedetomidine on spontaneous IPSCs which is blocked in the presence of spinal prazosin (an α1 antagonist, 10 μM) but not by yohimbine (an α2 antagonist, 4 μM).
These results suggest that the facilitatory action of systemic dexmedetomidine on spontaneous IPSCs may involve the descending noradrenergic system and spinal α1 adrenoceptors.
3.6 The effect of systemically administered dexmedetomidine on LC activity
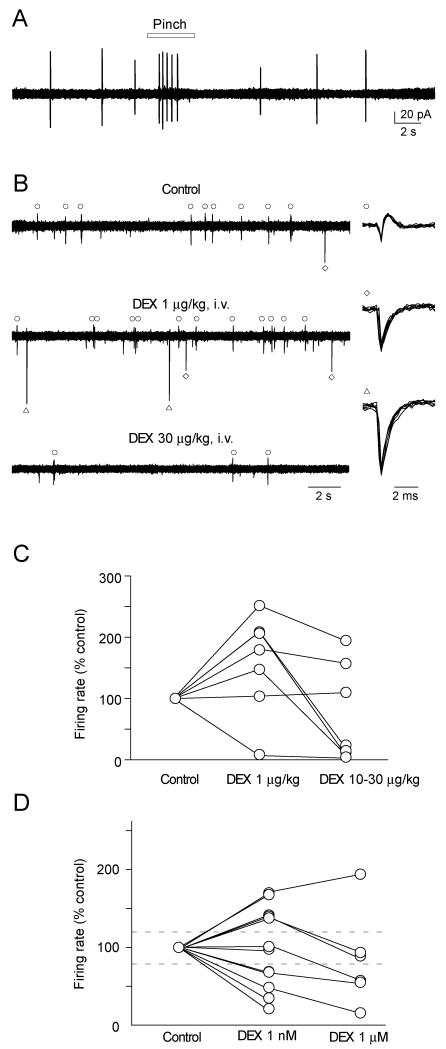
We next directly assessed whether systemic or locally applied dexmedetomidine could alter the excitability of LC neurons in vivo. Stable extracellular or cell-attached patch clamp recordings were obtained from a total of 21 LC neurons. These LC recordings showed the characteristic biphasic response to pinch stimulation applied to the contralateral hind limb (Fig. 6A) [59]. Their average spontaneous firing rates were 8.0 ± 3.1 Hz (n = 21).
Figure 6. Activation of LC neurons by low-dose systemic dexmedetomidine.
A) The characteristic biphasic response with excitation followed by inhibition of LC neuron firing by cutaneous pinch stimulation applied to the contralateral hind paw (cell-attached patch recording).
B) Extracellular recording showing several discriminated spontaneous LC units (right panels). For each cell the firing rate was increased by low dose systemic dexmedetomidine (1 μg/kg). However, higher doses of dexmedetomidine (30 μg/kg) strongly inhibited the firing of the same neurons.
C) Normalized firing rates of LC neurons after systemic dexmedetomidine (1-30 μg/kg). The firing rate was increased in 5 of 7 LC neurons at 1 μg/kg.
D) Normalized LC firing rates after superfusion of Dexmedetomidine over the floor of the 4th ventricle. Firing rates were increased in a substantial proportion of the cells (5/11) at 1nM Dexmedetomidine but the majority of the cells were inhibited by the 1uM dose. Dashed lines in C and D show 20% increase or decrease in normalized firing frequency.
During an ascending systemic dexmedetomidine dose protocol we observed that 5 out of the 7 LC neurons showed an increase (more than 20 % of control) in their firing frequency at low doses (139 ± 4 % (n = 5) at 0.01 μg/kg, 155 ± 35 % (n = 5) at 0.1 μg/kg, 200 ± 17 % (n = 5) at 1 μg/kg) which was reversed (and in some cases inhibited) at higher doses (78 ± 39 % of controls, n = 5, Fig. 6B, C; 10 - 30 μg/kg). These results indicate that systemic dexmedetomidine at lower doses activates LC neurons.
Next we applied dexmedetomidine at a concentrations of 1 nM onto the dorsal surface of the brain stem during LC recording. As shown in Fig. 6D, 4 out of 12 LC neurons showed a decrease (more than −20 % of control) in their firing rate, however 5 out of the LC neurons tested showed an increase (more than 20 % of control) in their firing rates, at 1 nM (150.9 ± 6.9 %). At 1 μM, dexmedetomidine inhibited the firing frequency in most (5 out of 6) LC neurons tested. These results suggest that dexmedetomidine at lower doses can activate some LC neurons.
4. Discussion
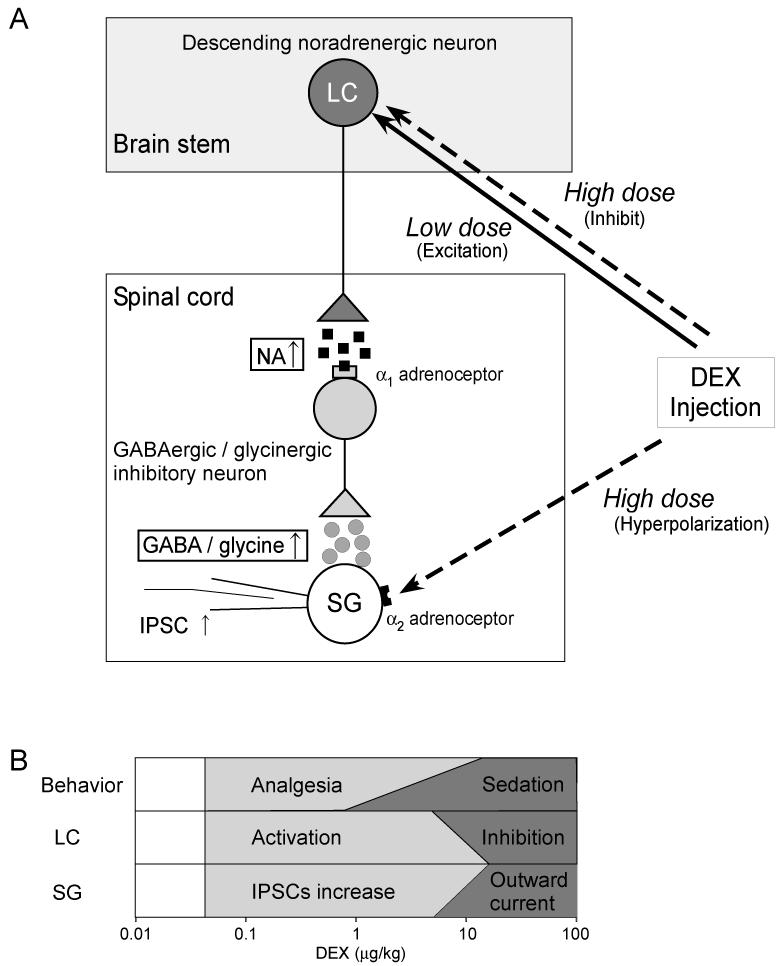
In this study, we have examined the antinociceptive action of systemic dexmedetomidine, the α2-adrenoceptor agonist, at a spinal level in vivo. We show that inhibitory synaptic transmission within the substantia gelatinosa was dramatically enhanced by low-dose dexmedetomidine. This action was mediated through a supraspinal mechanism involving the descending noradrenergic system at doses of dexmedetomidine that produce minimal sedation (see Fig. 7). Systemic administration of dexmedetomidine at low doses (0.01-10 μg/kg) activates the LC – a source of the descending noradrenergic projection. This activation of the descending noradrenergic pathway enhances spinal inhibitory transmission via α1-adrenoceptors not α2-adrenoceptors. Conversely, higher doses of dexmedetomidine (more than 10 μg/kg) strongly inhibit LC neurons (perhaps also producing the stronger sedative action) and simultaneously induces outward currents in spinal SG neurons via a direct postsynaptic α2-activation which probably produces additional analgesic effects (see Fig. 7B).
Figure 7. Schematics summarizing the proposed mechanisms of action of systemic dexmedetomidine.
A) Low dose systemic dexmedetomidine (DEX) facilitates spinal IPSCs by disinhibiting a noradrenergic descending pathway. The spinal noradrenaline (NA) acts via pre- and postsynaptic α1 adrenoceptors on the GABAergic and glycinergic inhibitory neurons to facilitate inhibitory synaptic transmission onto SG neurons. In contrast, high dose dexmedetomidine inhibits the LC, perhaps leading to sedation, and also directly activates α2-adrenoceptors on the SG neurons to induce outward potassium currents that hyperpolarize and inhibit the cells producing analgesia by a different mechanism.
B) Diagram showing the multiple actions of systemically administered dexmedetomidine at different doses. The analgesia and the facilitation of spontaneous IPSCs appear with low doses of systemic dexmedetomidine (0.1-1 μg/kg). At these doses dexmedetomidine enhanced the activity of LC neurons. Higher doses (10-75 μg/kg) of dexmedetomidine were needed to induce outward currents in SG neurons while also directly inhibiting the LC perhaps producing the known sedative effect.
We show that dexmedetomidine has analgesic actions on the withdrawal response to mechanical noxious stimuli (1-10 μg/kg). This compares to studies that have examined its action on thermal withdrawal latencies where it is effective at doses of 5-20 μg/kg [3; 18; 70]. Systemic dexmedetomidine produces a loss of righting reflex with an ED50 of ~16 μg/kg for intravenous bolus administration [54], at doses of >100 μg/kg following intraperitoneal administration [16] and sedative action on electroencephalogram at doses around 10 μg/kg [10] and a decrease in spontaneous locomotive activity at doses of >30 μg/kg [56]. Our sedative assessment showed that dexmedetomidine has detectable sedative actions at doses of 10 μg/kg and minimal sedation was detected at a dose of 1 μg/kg, but that moderate to heavy sedation was only seen at doses of 30 μg/kg. This suggests that there may be a dissociation between the dose range over which the mechanical analgesia and the clinically relevant sedative effects can be elicited by systemic dexmedetomidine. Given that mechanical analgesia was observed over lower dose range than thermal analgesia under light sedation [3; 18; 70], it raises the possibility that it may be mediated through a different cellular/molecular mechanism from those previously documented.
Based on previous in vitro studies, we hypothesized that low-dose dexmedetomidine (<10 μg/kg) would act either to directly inhibit SG neurons by activation of a potassium conductance [25; 31; 49; 58] or by presynaptic inhibition of excitatory transmission [37; 53; 58]. However, despite searching specifically for these actions, we only found outward currents at high doses (≥30 μg/kg) and never saw any effect on spontaneous excitatory synaptic transmission. A previous study demonstrated that dexmedetomidine has direct spinal effects in neuropathic pain models at intrathecal doses which are ineffective in normal rats [40]. However, we found dexmedetomidine (1 μg/kg) did not induce outward currents in SG neurons in allodynic rats. Previous behavioral and in vivo studies showed that intrathecally administered dexmedetomidine has an antinociceptive action and produced a decrease in spinal neural activity via α2-adrenoceptors [34; 60; 69]. Such direct spinal actions to induce outward currents [31] was only seen at high doses in our study but would be expected to produce strong analgesic actions (see also Fig. 7).
Our observation of a lack of effect of dexmedetomidine on spontaneous EPSCs is consistent with previous in vitro studies showing that noradrenaline had no effect on spontaneous EPSCs recorded in SG neurons [31; 37] but was able to suppress primary afferent evoked EPSCs. To accurately elucidate the presynaptic effect of dexmedetomidine would require electrophysiological experiments to assess paired-pulse ratio and the coefficient of variation of evoked EPSCs using spinal cord slice. However, these experiments cannot currently be performed in our preparation in vivo so we aimed to test for a presynaptic effect of systemic dexmedetomidine by recording miniature EPSCs in the presence of TTX. Systemic dexmedetomidine had no effect on miniature EPSC frequency or amplitude implying that it is not acting presynaptically to modulate excitatory transmission. Taken together these results suggested that systemic dexmedetomidine at low doses produces antinociception by a novel mechanism.
Counter to our expectations, we found low doses of systemic dexmedetomidine enhanced spontaneous IPSCs in most SG neurons (~90 %). The enhancement of spinal IPSCs by systemic dexmedetomidine was lost in spinalized animals and, importantly, was not mimicked by direct spinal application of dexmedetomidine (0.1 – 10 μM). These findings suggested that this dexmedetomidine-induced enhancement of spinal IPSCs may be dependent upon a descending pathway from the pons [28; 32; 50]. A similar IPSC facilitation by noradrenaline has been shown to be present in most (~90 %) SG neurons in spinal cord slices in vitro [5; 6] mediated by α1-adrenoceptors. It has further been shown that α1-adrenoceptors are expressed in the superficial dorsal horn [27; 62] and that noradrenaline acts to excite spinal GABAergic neurons via α1-adrenoceptors [23]. We demonstrated this effect in 83% of SG neurons tested in vivo with direct spinal superfusion of noradrenaline. Almost all of the SG neurons that responded in this way to noradrenaline also showed facilitation of IPSCs by systemic dexmedetomidine. Furthermore this systemic action of dexmedetomidine on IPSCs was blocked by spinal application of the α1-adrenoceptor antagonist prazosin, but not by the α2-adrenoceptor antagonist yohimbine.
As we have discussed already spinal noradrenaline can exert antinociceptive actions by several mechanisms; facilitation of inhibitory synaptic responses via α1 adrenoceptors, presynaptic inhibition of excitatory synaptic responses and hyperpolarization of SG via α2-adrenoceptors [50; 58]. Our in vivo patch clamp recordings indicate that the facilitation of inhibitory synaptic responses occurs to alter nociceptive transmission before the other direct spinally mediated α2-adrenoceptor actions. However the reason for this sensitivity/specificity and apparent dose threshold difference is still not known, we hope to perform more experiments to explore this interesting phenomenon.
The serotonergic pathways form another well-characterized descending monoaminergic projection to the spinal dorsal horn from the brain stem, [45; 65]. Previous patch-clamp recordings from adult spinal cord slices showed that bath-application of serotonin also facilitated inhibitory synaptic responses in SG neurons, although this serotonin-induced facilitation of IPSCs was found in a lower proportion (~50 %) of recordings [1; 64]. This suggests that both noradrenergic and serotoninergic descending control systems may act through a common mechanism to regulate the tonic level of inhibition impinging upon the SG. The spinal processing of nociceptive signals is known to be held under tonic regulation by GABA/glycinergic IPSCs [57] and that the loss of such inhibition is found in chronic pain models [46]. We speculate that the regulation of inhibitory GABA/glycinergic tone may be one of the principal means by which descending monoaminergic systems regulate nociceptive transmission in the SG.
Although we show that low-dose systemic dexmedetomidine can increase the firing rate of LC neurons in vivo, as can low concentrations of dexmedetomidine applied to the dorsal pons over the LC, this is somewhat paradoxical as it has been reported that α2-agonists inhibit LC neurons to exert sedative actions [2; 12; 15; 33]. In line with this finding, we noted that high doses of systemic dexmedetomidine inhibited LC activity. Interestingly a previous behavioral study also showed that injection of dexmedetomidine into the LC produced antinociception by activating a descending noradrenergic inhibitory pathway [26] but these authors suggested that this might be mediated indirectly via the A5 noradrenergic cell group. On the basis of our findings we propose that this analgesic effect may be caused by a disinhibition of the LC by low dose dexmedetomidine leading to an increase in noradrenaline release at a spinal level. This may be via a selective presynaptic disinhibition of the LC (which is known to receive tonic inhibition). It is also worth noting that most α2-agonists have affinity for imidazoline receptors [29] which are highly expressed in the Nucleus Paragigantocellullaris (PGi) in the ventral medulla. The LC is known to receive excitatory afferents from the PGi [17], and Pineda et al. have demonstrated that systemically administered high dose clonidine can paradoxically increase LC firing rate via an activation of imidazoline receptors. Further experiments will be needed to elucidate how LC neurons are excited by low doses of dexmedetomidine.
In conclusion, we have revealed a novel antinociceptive mechanism for systemic α2-adrenoceptor agonists at low doses via the facilitation of inhibitory synaptic transmission in the spinal dorsal horn. This is mediated by an activation of the descending noradrenergic inhibitory system originating from the LC in the pons which is acting to modulate inhibitory tone in the superficial dorsal horn of the spinal cord. This occurs at doses that are below the normal sedative range and by targeting this mechanism it may be possible to usefully produce analgesia with only minimal sedation, potentially extending the therapeutic utility of α2-agonists as analgesics. Further this potentiation of inhibitory synaptic transmission may also in part account for the known synergy between anesthetic agents that themselves potentiate inhibitory synaptic transmission (via a GABAA receptor mediated mechanism) and dexmedetomidine.
Acknowledgements
We would like to thank Dr. Kazuhiko Seki, and Ms. Hiromi Ishihara for their helpful advices on data analysis and technical support. This work was supported by grants from the programs Grants-in-Aid for Scientific Research (H. F. and K.I.) of the Ministry of Education, Science, Sports and Culture of Japan. AEP is a Welcome Trust Senior Clinical Research fellow.
Footnotes
Conflict of interest statement: There are no financial or other relationships that may cause a conflict of interest.
References
- [1].Abe K, Kato G, Katafuchi T, Tamae A, Furue H, Yoshimura M. Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience. 2009;159(1):316–324. doi: 10.1016/j.neuroscience.2008.12.021. [DOI] [PubMed] [Google Scholar]
- [2].Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215(4538):1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- [3].Asano T, Dohi S, Ohta S, Shimonaka H, Iida H. Antinociception by epidural and systemic alpha(2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. Anesthesia and Analgesia. 2000;90(2):400–407. doi: 10.1097/00000539-200002000-00030. [DOI] [PubMed] [Google Scholar]
- [4].Aston-Jones G. Locus Ceruleus, A5 and A7 Noradrenergic Cell Groups. In: Paxinoa G, editor. The Rat Nervous System. Elesevier Academic Press; San Diego, London: 2004. pp. 259–294. [Google Scholar]
- [5].Baba H, Goldstein PA, Okamoto M, Kohno T, Ataka T, Yoshimura M, Shimoji K. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): effects on somatodendritic sites of GABAergic neurons. Anesthesiology. 2000;92(2):485–492. doi: 10.1097/00000542-200002000-00031. [DOI] [PubMed] [Google Scholar]
- [6].Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473–484. doi: 10.1097/00000542-200002000-00030. [DOI] [PubMed] [Google Scholar]
- [7].Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci. 2004;24(20):4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Annals of Neurology. 1978;4(5):451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- [9].Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116(6):1312–1322. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- [10].Bol C, Danhof M, Stanski DR, Mandema JW. Pharmacokinetic-pharmacodynamic characterization of the cardiovascular, hypnotic, EEG and ventilatory responses to dexmedetomidine in the rat. J Pharmacol Exp Ther. 1997;283(3):1051–1058. [PubMed] [Google Scholar]
- [11].Cervero F, Iggo A. The substantia gelatinosa of the spinal cord: a critical review. Brain. 1980;103(4):717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- [12].Chiu TH, Chen MJ, Yang YR, Yang JJ, Tang FI. Action of dexmedetomidine on rat locus coeruleus neurones: intracellular recording in vitro. European Journal of Pharmacology. 1995;285(3):261–268. doi: 10.1016/0014-2999(95)00417-j. [DOI] [PubMed] [Google Scholar]
- [13].Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol. 2008;4(5):619–627. doi: 10.1517/17425255.4.5.619. [DOI] [PubMed] [Google Scholar]
- [14].Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79(2):154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- [15].Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76(6):948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- [16].Doze VA, Chen BX, Maze M. Dexmedetomidine produces a hypnotic-anesthetic action in rats via activation of central alpha-2 adrenoceptors. Anesthesiology. 1989;71(1):75–79. doi: 10.1097/00000542-198907000-00014. [DOI] [PubMed] [Google Scholar]
- [17].Ennis M, Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J Neurosci. 1988;8(10):3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fisher B, Zornow MH, Yaksh TL, Peterson BM. Antinociceptive properties of intrathecal dexmedetomidine in rats. European Journal of Pharmacology. 1991;192(2):221–225. doi: 10.1016/0014-2999(91)90046-s. [DOI] [PubMed] [Google Scholar]
- [19].Furue H. In Vivo Blind Patch-Clamp Recording Technique. In: Okada Y, editor. Patch-ClampTechniques. Springer; Tokyo, London, New York: 2012. pp. 171–182. [Google Scholar]
- [20].Furue H, Katafuchi T, Yoshimura M. Sensory processing and functional reorganization of sensory transmission under pathological conditions in the spinal dorsal horn. Neurosci Res. 2004;48(4):361–368. doi: 10.1016/j.neures.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [21].Furue H, Katafuchi T, Yoshimura M. In Vivo Patch-Clamp Technique. In: Walz W, editor. Patch-Clamp Analysis Advanced Techniques. Humana Press; Totowa: 2007. pp. 229–251. [Google Scholar]
- [22].Furue H, Narikawa K, Kumamoto E, Yoshimura M. Responsiveness of rat substantia gelatinosa neurones to mechanical but not thermal stimuli revealed by in vivo patch-clamp recording. J Physiol. 1999;521(Pt 2):529–535. doi: 10.1111/j.1469-7793.1999.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gassner M, Ruscheweyh R, Sandkuhler J. Direct excitation of spinal GABAergic interneurons by noradrenaline. Pain. 2009;145(1-2):204–210. doi: 10.1016/j.pain.2009.06.021. [DOI] [PubMed] [Google Scholar]
- [24].Gilsbach R, Hein L. Are the pharmacology and physiology of α2 adrenoceptors determined by α2-heteroreceptors and autoreceptors respectively? British Journal of Pharmacolology. 2012;165(1):90–102. doi: 10.1111/j.1476-5381.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grudt TJ, Williams JT, Travagli RA. Inhibition by 5-hydroxytryptamine and noradrenaline in substantia gelatinosa of guinea-pig spinal trigeminal nucleus. J Physiol. 1995;485(Pt 1):113–120. doi: 10.1113/jphysiol.1995.sp020716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84(4):873–881. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- [27].Hagihira S, Senba E, Yoshida S, Tohyama M, Yoshiya I. Fine structure of noradrenergic terminals and their synapses in the rat spinal dorsal horn: an immunohistochemical study. Brain Research. 1990;526(1):73–80. doi: 10.1016/0006-8993(90)90251-6. [DOI] [PubMed] [Google Scholar]
- [28].Hayashida K, Eisenach JC. Spinal alpha 2-adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function. Anesthesiology. 2010;113(2):406–412. doi: 10.1097/ALN.0b013e3181de6d2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hieble JP, Ruffolo RR., Jr. Possible structural and functional relationships between imidazoline receptors and alpha 2-adrenoceptors. Ann N Y Acad Sci. 1995;763:8–21. doi: 10.1111/j.1749-6632.1995.tb32387.x. [DOI] [PubMed] [Google Scholar]
- [30].Howorth PW, Thornton SR, O’Brien V, Smith WD, Nikiforova N, Teschemacher AG, Pickering AE. Retrograde viral vector-mediated inhibition of pontospinal noradrenergic neurons causes hyperalgesia in rats. J Neurosci. 2009;29(41):12855–12864. doi: 10.1523/JNEUROSCI.1699-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ishii H, Kohno T, Yamakura T, Ikoma M, Baba H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. European Journal of Neuroscience. 2008;27(12):3182–3190. doi: 10.1111/j.1460-9568.2008.06260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jones SL. Descending noradrenergic influences on pain. Progress in Brain Research. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- [33].Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. British Journal of Anaesthesia. 1993;71(3):447–449. doi: 10.1093/bja/71.3.447. [DOI] [PubMed] [Google Scholar]
- [34].Kalso EA, Poyhia R, Rosenberg PH. Spinal antinociception by dexmedetomidine, a highly selective alpha 2-adrenergic agonist. Pharmacol Toxicol. 1991;68(2):140–143. doi: 10.1111/j.1600-0773.1991.tb02052.x. [DOI] [PubMed] [Google Scholar]
- [35].Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93(5):1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- [36].Kato G, Yasaka T, Katafuchi T, Furue H, Mizuno M, Iwamoto Y, Yoshimura M. Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats. J Neurosci. 2006;26(6):1787–1794. doi: 10.1523/JNEUROSCI.4856-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682–689. doi: 10.1097/00000542-200303000-00016. [DOI] [PubMed] [Google Scholar]
- [38].Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54(2):146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- [39].Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- [40].Kimura M, Saito S, Obata H. Dexmedetomidine decreases hyperalgesia in neuropathic pain by increasing acetylcholine in the spinal cord. Neurosci Lett. 529(1):70–74. doi: 10.1016/j.neulet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- [41].Kohno T, Kumamoto E, Baba H, Ataka T, Okamoto M, Shimoji K, Yoshimura M. Actions of midazolam on GABAergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Anesthesiology. 2000;92(2):507–515. doi: 10.1097/00000542-200002000-00034. [DOI] [PubMed] [Google Scholar]
- [42].Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978;177(3):417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- [43].Kwiat GC, Basbaum AI. The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somatosensory and Motor Research. 1992;9(2):157–173. doi: 10.3109/08990229209144768. [DOI] [PubMed] [Google Scholar]
- [44].Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186(2):133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- [45].Millan MJ. Descending control of pain. Progress in Neurobiology. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- [46].Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22(15):6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nakatsuka T, Furue H, Yoshimura M, Gu JG. Activation of central terminal vanilloid receptor-1 receptors and alpha beta-methylene-ATP-sensitive P2X receptors reveals a converged synaptic activity onto the deep dorsal horn neurons of the spinal cord. J Neurosci. 2002;22(4):1228–1237. doi: 10.1523/JNEUROSCI.22-04-01228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Narikawa K, Furue H, Kumamoto E, Yoshimura M. In vivo patch-clamp analysis of IPSCs evoked in rat substantia gelatinosa neurons by cutaneous mechanical stimulation. J Neurophysiol. 2000;84(4):2171–2174. doi: 10.1152/jn.2000.84.4.2171. [DOI] [PubMed] [Google Scholar]
- [49].North RA, Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 1984;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80(2):53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [51].Pineda J, Ugedo L, Garcia-Sevilla JA. Stimulatory effects of clonidine, cirazoline and rilmenidine on locus coeruleus noradrenergic neurones: possible involvement of imidazoline-preferring receptors. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(2):134–140. doi: 10.1007/BF00164789. [DOI] [PubMed] [Google Scholar]
- [52].Ruffolo RR, Jr., Nichols AJ, Stadel JM, Hieble JP. Structure and function of alpha-adrenoceptors. Pharmacological Reviews. 1991;43(4):475–505. [PubMed] [Google Scholar]
- [53].Ruscheweyh R, Sandkuhler J. Differential actions of spinal analgesics on mono-versus polysynaptic Adelta-fibre-evoked field potentials in superficial spinal dorsal horn in vitro. Pain. 2000;88(1):97–108. doi: 10.1016/S0304-3959(00)00325-0. [DOI] [PubMed] [Google Scholar]
- [54].Salonen M, Reid K, Maze M. Synergistic interaction between alpha 2-adrenergic agonists and benzodiazepines in rats. Anesthesiology. 1992;76(6):1004–1011. doi: 10.1097/00000542-199206000-00022. [DOI] [PubMed] [Google Scholar]
- [55].Sandkuhler J. The organization and function of endogenous antinociceptive systems. Progress in Neurobiology. 1996;50(1):49–81. doi: 10.1016/0301-0082(96)00031-7. [DOI] [PubMed] [Google Scholar]
- [56].Seppala T, Idanpaan-Heikkila JJ, Stromberg C, Mattila MJ. Ethanol antagonism by atipamezole on motor performance in mice. Life Sci. 1994;55(3):245–251. doi: 10.1016/0024-3205(94)00886-8. [DOI] [PubMed] [Google Scholar]
- [57].Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72(1):169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- [58].Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol. 2004;555(Pt 2):515–526. doi: 10.1113/jphysiol.2003.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sugiyama D, Hur SW, Pickering AE, Kase D, Kim SJ, Kawamata M, Imoto K, Furue H. In vivo patch-clamp recording from locus coeruleus neurones in the rat brainstem. J Physiol. 2012;590(Pt 10):2225–2231. doi: 10.1113/jphysiol.2011.226407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sullivan AF, Kalso EA, McQuay HJ, Dickenson AH. The antinociceptive actions of dexmedetomidine on dorsal horn neuronal responses in the anaesthetized rat. Eur J Pharmacol. 1992;215(1):127–133. doi: 10.1016/0014-2999(92)90617-d. [DOI] [PubMed] [Google Scholar]
- [61].Takano Y, Yaksh TL. Relative efficacy of spinal alpha-2 agonists, dexmedetomidine, clonidine and ST-91, determined in vivo by using N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline, an irreversible antagonist. J Pharmacol Exp Ther. 1991;258(2):438–446. [PubMed] [Google Scholar]
- [62].Wada T, Otsu T, Hasegawa Y, Mizuchi A, Ono H. Characterization of alpha 1-adrenoceptor subtypes in rat spinal cord. Eur J Pharmacol. 1996;312(2):263–266. doi: 10.1016/0014-2999(96)00570-5. [DOI] [PubMed] [Google Scholar]
- [63].Woolf CJ, Fitzgerald M. The properties of neurones recorded in the superficial dorsal horn of the rat spinal cord. J Comp Neurol. 1983;221(3):313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]
- [64].Xie DJ, Uta D, Feng PY, Wakita M, Shin MC, Furue H, Yoshimura M. Identification of 5-HT receptor subtypes enhancing inhibitory transmission in the rat spinal dorsal horn in vitro. Mol Pain. 2012;8:58. doi: 10.1186/1744-8069-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci. 2006;101(2):107–117. doi: 10.1254/jphs.crj06008x. [DOI] [PubMed] [Google Scholar]
- [66].Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62(1):96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- [67].Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53(2):519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]
- [68].Yoshimura M, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro. J Physiol. 1995;482(Pt 1):29–38. doi: 10.1113/jphysiol.1995.sp020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang H, Zhou F, Li C, Kong M, Liu H, Zhang P, Zhang S, Cao J, Zhang L, Ma H. Molecular mechanisms underlying the analgesic property of intrathecal dexmedetomidine and its neurotoxicity evaluation: an in vivo and in vitro experimental study. PLoS One. 8(2):e55556. doi: 10.1371/journal.pone.0055556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang WS, Xu H, Xu B, Sun S, Deng XM, Zhang YQ. Antihyperalgesic effect of systemic dexmedetomidine and gabapentin in a rat model of monoarthritis. Brain Research. 2009;1264:57–66. doi: 10.1016/j.brainres.2009.01.029. [DOI] [PubMed] [Google Scholar]