Fig. 2.
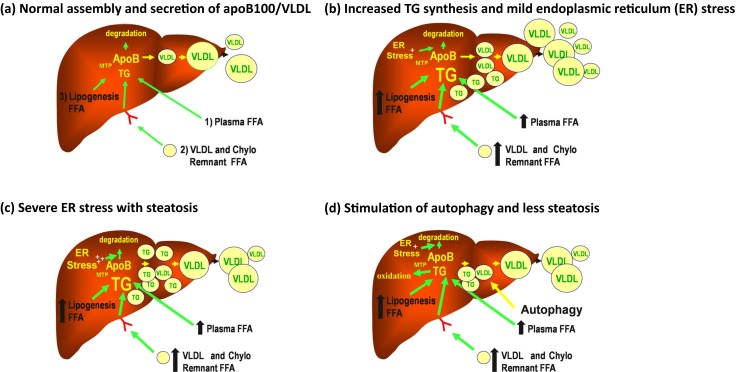
Forces regulating the assembly and secretion of apoB100/VLDL. ApoB100/VLDL is a vehicle for transporting energy from the liver to the periphery and its assembly and secretion are extremely complex. a Nascent lipid-poor apoB100 is either targeted for complete translocation into the lumen of the endoplasmic reticulum (ER) for assembly into VLDL or for co- and post-translational ER degradation. The fate of nascent apoB is dependent on lipidation with TG via the action of microsomal triglyceride transfer protein (MTP). The TG originates from free fatty acids (FFA) derived from three sources: 1) lipolysis of adipose tissue, 2) VLDL and chylomicron remnants returning to the liver, and 3) hepatic de novo lipogenesis. The VLDL formed in this step then matures with addition of more core lipids, principally TG. This second step is also regulated by MTP and occurs either in the ER or in a post-ER compartment, such as the Golgi. At this point, mature VLDL can be secreted. b When TG synthesis from any source is increased, the response is an increase in VLDL secretion. However, mild or moderate ER stress can also result. ER stress can cause increased degradation of apoB leading to less than maximal VLDL secretion and some steatosis. c If ER stress becomes even more severe, degradation of apoB increases further and VLDL secretion can fall even more, leading to significant steatosis. d Under certain conditions, autophagy can be induced at this point and VLDL can be diverted for post-ER/Golgi degradation. Lipophagy and ER autophagy may also occur, resulting in increased fatty acid oxidation and less steatosis. Thus autophagy might promote both less hepatic steatosis and less apoB100/VLDL secretion
