Abstract
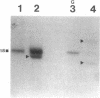
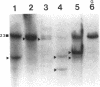
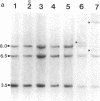
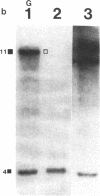
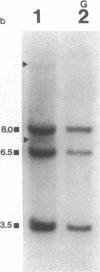
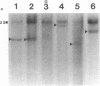
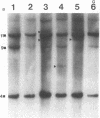
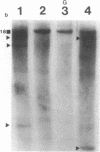
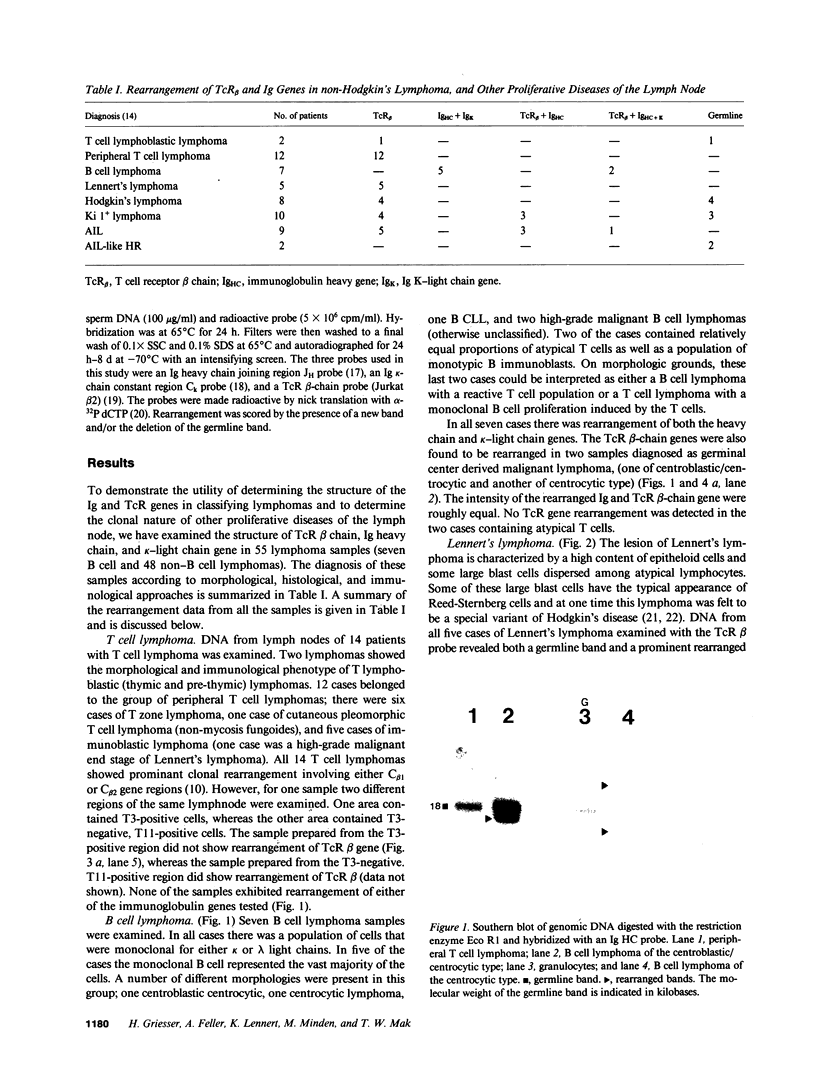
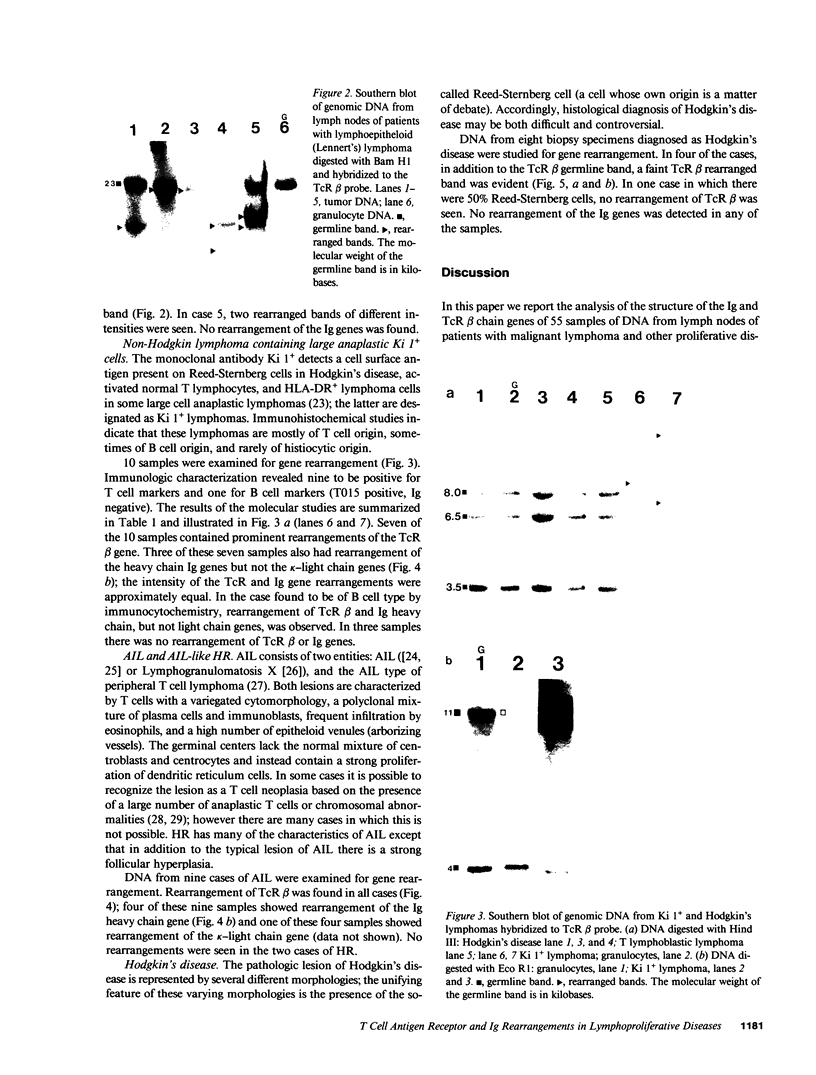
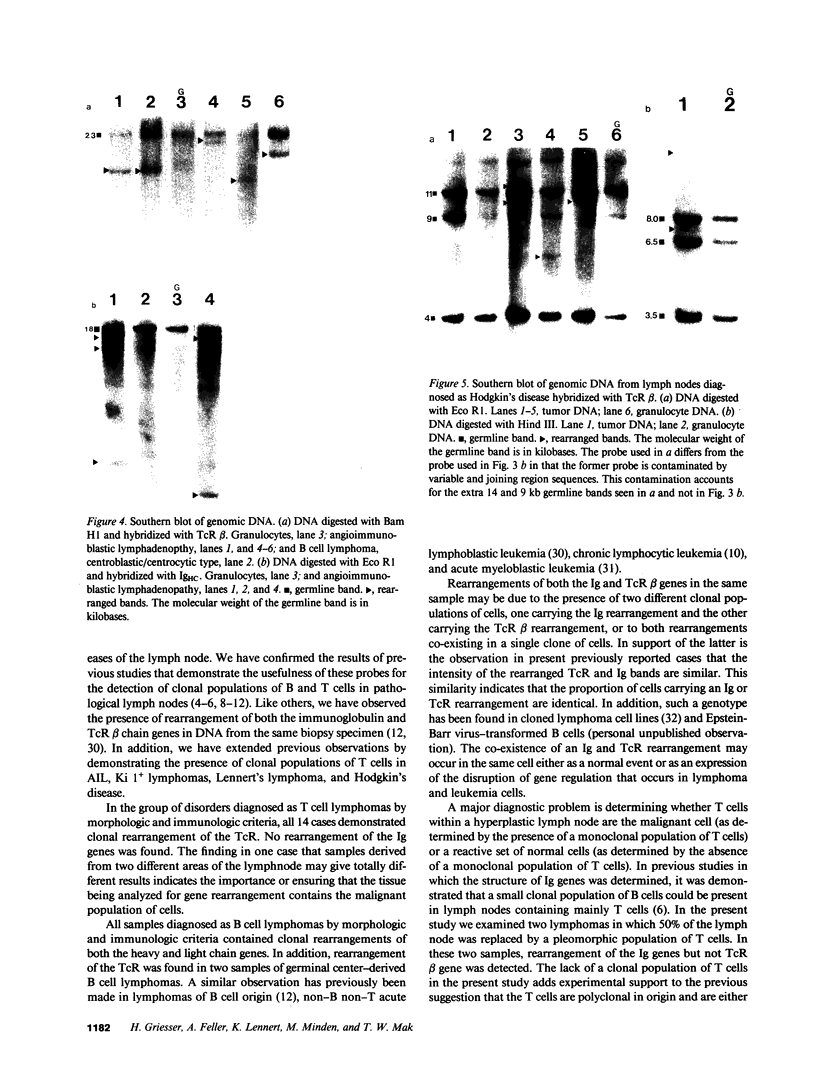
55 samples representing Hodgkin's and non-Hodgkin's lymphoma and other hyperplastic lesions of the lymph node were examined for rearrangement of the beta chain of the T cell antigen receptor (TcR) and Ig genes. In non-Hodgkin's lymphoma, rearrangement of TcR beta was found in all 14 T cell lymphomas and in two of the seven B cell lymphomas. Ig gene rearrangement was found in none of the 14 T cell lymphomas and in all seven B cell lymphomas. We also examined DNA from lymph nodes in which the lineage of the malignant cell is not clear. Rearrangement of TcR beta was found in all five lymphoepitheloid cell (Lennert's) lymphomas; four of eight Hodgkin's lymphomas; seven of ten Ki 1+ lymphomas; and all nine cases of angioimmunoblastic lymphoadenopathy (AIL). Ig gene rearrangement was found in none of five lymphoepitheloid cell lymphomas; none of eight Hodgkin's lymphomas; three of ten Ki 1+ lymphomas; and four of nine cases of AIL. These findings indicate that genetic studies of TcR and Ig genes are useful in identifying the presence of a clonal population in a lymph node, in determining the extent of the clonal population, and aid in identifying lineage. Of special interest was the finding that some cases of Hodgkin's lymphoma and AIL contain clonal rearrangement of the TcR genes, which suggests that in those cases the malignant cells may be of T cell origin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Cossman J., Bakhshi A., Jaffe E. S., Waldmann T. A., Korsmeyer S. J. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983 Dec 29;309(26):1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- Cheng G. Y., Minden M. D., Toyonaga B., Mak T. W., McCulloch E. A. T cell receptor and immunoglobulin gene rearrangements in acute myeloblastic leukemia. J Exp Med. 1986 Feb 1;163(2):414–424. doi: 10.1084/jem.163.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzera G., Moran E. M., Rappaport H. Angio-immunoblastic lymphadenopathy with dysproteinaemia. Lancet. 1974 Jun 1;1(7866):1070–1073. doi: 10.1016/s0140-6736(74)90553-4. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Keys C., VarsanyiBreiner A., Kao F. T., Jones C., Puck T. T., Housman D. Isolation and localization of DNA segments from specific human chromosomes. Proc Natl Acad Sci U S A. 1980 May;77(5):2829–2833. doi: 10.1073/pnas.77.5.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gödde-Salz E., Schwarze E. W. Biclonal trisomy 3 in a case of epithelioid cellular lymphogranulomatosis (Lennert's lymphoma). Cancer Genet Cytogenet. 1984 Dec;13(4):337–341. doi: 10.1016/0165-4608(84)90077-3. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Max E. E., Seidman J. G., Maizel J. V., Jr, Leder P. Cloned human and mouse kappa immunoglobulin constant and J region genes conserve homology in functional segments. Cell. 1980 Nov;22(1 Pt 1):197–207. doi: 10.1016/0092-8674(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Hossfeld D. K., Höffken K., Schmidt C. G., Diedrichs H. Letter: Chromosome abnormalities in angioimmunoblastic lymphadenopathy. Lancet. 1976 Jan 24;1(7952):198–198. doi: 10.1016/s0140-6736(76)91308-8. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Larson R. A., Variakojis D., Haren J. M., Rowley J. D. Nonrandom chromosome abnormalities in angioimmunoblastic lymphadenopathy. Blood. 1982 Oct;60(4):877–887. [PubMed] [Google Scholar]
- Kitchingman G. R., Rovigatti U., Mauer A. M., Melvin S., Murphy S. B., Stass S. Rearrangement of immunoglobulin heavy chain genes in T cell acute lymphoblastic leukemia. Blood. 1985 Mar;65(3):725–729. [PubMed] [Google Scholar]
- Knecht H., Schwarze E. W., Lennert K. Histological, immunohistological and autopsy findings in lymphogranulomatosis X (including angio-immunoblastic lymphadenopathy). Virchows Arch A Pathol Anat Histopathol. 1985;406(1):105–124. doi: 10.1007/BF00710561. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Arnold A., Bakhshi A., Ravetch J. V., Siebenlist U., Hieter P. A., Sharrow S. O., LeBien T. W., Kersey J. H., Poplack D. G. Immunoglobulin gene rearrangement and cell surface antigen expression in acute lymphocytic leukemias of T cell and B cell precursor origins. J Clin Invest. 1983 Feb;71(2):301–313. doi: 10.1172/JCI110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Lennert K., Mestdagh J. Lymphogranulomatosen mit konstant hohem Epitheloidzellgehalt. Virchows Arch A Pathol Pathol Anat. 1968;344(1):1–20. [PubMed] [Google Scholar]
- Lukes R. J., Parker J. W., Taylor C. R., Tindle B. H., Cramer A. D., Lincoln T. L. Immunologic approach to non-Hodgkin lymphomas and related leukemias. Analysis of the results of multiparameter studies of 425 cases. Semin Hematol. 1978 Oct;15(4):322–351. [PubMed] [Google Scholar]
- Lukes R. J., Tindle B. H. Immunoblastic lymphadenopathy. A hyperimmune entity resembling Hodgkin's disease. N Engl J Med. 1975 Jan 2;292(1):1–8. doi: 10.1056/NEJM197501022920101. [DOI] [PubMed] [Google Scholar]
- Minden M. D., Toyonaga B., Ha K., Yanagi Y., Chin B., Gelfand E., Mak T. Somatic rearrangement of T-cell antigen receptor gene in human T-cell malignancies. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1224–1227. doi: 10.1073/pnas.82.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor N. T., Wainscoat J. S., Weatherall D. J., Gatter K. C., Feller A. C., Isaacson P., Jones D., Lennert K., Pallesen G., Ramsey A. Rearrangement of the T-cell-receptor beta-chain gene in the diagnosis of lymphoproliferative disorders. Lancet. 1985 Jun 8;1(8441):1295–1297. doi: 10.1016/s0140-6736(85)92791-6. [DOI] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Dalla Favera R. Lymphoid tumors displaying rearrangements of both immunoglobulin and T cell receptor genes. J Exp Med. 1985 Sep 1;162(3):1015–1024. doi: 10.1084/jem.162.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaszkiewicz T., Lennert K. Lymphogranulomatosis X. Klinisches Bild, Therapie und Prognose. Dtsch Med Wochenschr. 1975 May 23;100(21):1157–1163. doi: 10.1055/s-0028-1106350. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Siegelman M. H., Cleary M. L., Warnke R., Sklar J. Frequent biclonality and Ig gene alterations among B cell lymphomas that show multiple histologic forms. J Exp Med. 1985 Apr 1;161(4):850–863. doi: 10.1084/jem.161.4.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein H., Lennert K., Feller A. C., Mason D. Y. Immunohistological analysis of human lymphoma: correlation of histological and immunological categories. Adv Cancer Res. 1984;42:67–147. doi: 10.1016/s0065-230x(08)60456-x. [DOI] [PubMed] [Google Scholar]
- Stein H., Lennert K., Feller A. C., Mason D. Y. Immunohistological analysis of human lymphoma: correlation of histological and immunological categories. Adv Cancer Res. 1984;42:67–147. doi: 10.1016/s0065-230x(08)60456-x. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Tawa A., Hozumi N., Minden M., Mak T. W., Gelfand E. W. Rearrangement of the T-cell receptor beta-chain gene in non-T-cell, non-B-cell acute lymphoblastic leukemia of childhood. N Engl J Med. 1985 Oct 24;313(17):1033–1037. doi: 10.1056/NEJM198510243131701. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., McIntyre P. A. Stimulation by propylthiouracil of the hexose monophosphate shunt in human polymorphonuclear leucocytes during phagocytosis. Br J Haematol. 1975 Oct;31(2):193–208. doi: 10.1111/j.1365-2141.1975.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Davis M. M., Bongiovanni K. F., Korsmeyer S. J. Rearrangements of genes for the antigen receptor on T cells as markers of lineage and clonality in human lymphoid neoplasms. N Engl J Med. 1985 Sep 26;313(13):776–783. doi: 10.1056/NEJM198509263131303. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]