Abstract
Purpose of Review
The field of prostate cancer therapeutics has undergone a rapid and dramatic change in the last few years. Multiple agents with very distinct mechanisms of actions and unique toxicities and efficacies have become available for clinical use. The focus of this review is to give a summary of clinical perspectives of the indications, including pros and cons of the currently approved regimens. The next generation of novel targets and agents are also highlighted.
Recent Findings
Addition of docetaxel based chemotherapy to conventional androgen suppression therapy in hormone sensitive advanced prostate cancer demonstrated overall survival benefit in recently released results of ECOG 3805. In castrate resistant metastatic disease, development of novel immunotherapy (Sipuleucel T), chemotherapy (docetaxel and cabazitaxel), radiation (alpharadin) and hormone therapy (abiraterone and enzalutamide) agents has created a range of choices for treatment, palliation and improved life expectancy.
Summary
A paradigm shift has occurred in the management of advanced prostate cancer, with multiple novel agents addressing distinct pathways, and demonstrating powerful efficacy. The judicious use of the available agents, with finesse of sequencing, and concomitant palliative care has prolonged survival and made living with the disease more reasonable and tolerable.
Keywords: Prostate cancer, metastatic, castrate resistant, systemic therapies
Introduction
Recent advances in systemic therapy have altered the landscape of metastatic prostate cancer. New paradigms of therapy have emerged and as a result, the natural history of prostate cancer is undergoing rapid transformation. Multiple agents with very distinct mechanisms of actions and unique toxicities and efficacies have made the field particularly overwhelming. A lot of these changes have also occurred in a very short time interval of the last 3–5 years. Even the multiple reviews written in the last few years are rapidly outdated as more trials get reported. The focus of this review is to give a summary of the current clinical perspectives of the indications, including pros and cons of the currently approved regimens.
Androgen deprivation therapy remains the mainstay of front line therapy for advanced prostate cancer. Nevertheless, this treatment is not curative and patients invariably develop progressive disease. Castrate resistant prostate cancer (CRPC) implies any disease progression (PSA only progression, or progression of existing metastases, or development of new metastasis) despite being on adequate androgen deprivation therapy. The field of advanced prostate cancer, especially in the castrate resistant setting, has undergone a rapid overhaul in the last five years. A paradigm shift has occurred, with the availability of multiple novel agents addressing distinct pathways, and demonstrating greater efficacy, in a disease state which previously had a large unmet need.
The judicious use of multiple available agents, with finesse of sequencing, and concomitant palliative care, has prolonged overall survival (OS) and made living with metastatic CRPC a more reasonable and tolerable experience than previously. The expertise of multiple specialties can ensure an optimal outcome, not only in terms of improving life expectancy but helping to lead a full productive life. To deliver the variety of treatments ranging from immunotherapy to bone targeted radiation therapy, it is imperative to involve multiple specialties in the care of the advanced prostate cancer patient. In addition, the psychological reassurance to patients and families of having continued help and support available from multiple specialties can never be underestimated.
At present the choice of therapies is predominantly based on clinical prognostic markers, such as patient and tumor characteristics. Development of predictive markers is a dire need in this disease. This will help optimize therapies, reduce unnecessary toxicities and reduce costs. Overcoming resistance to currently available therapies is an area of active research. Identification of novel targets of attack, and agents directed towards these targets, represent ongoing avenues of investigation.
Therapeutic Options in Untreated Metastatic Prostate Cancer
Conventional androgen suppression therapy remains the mainstay of systemic front line therapies in metastatic prostate cancer. In metastatic prostate cancer, orchiectomy has gradually been replaced by chemical castration methods with LHRH analogues or LHRH antagonists. Interestingly, although the addition of anti-androgens to orchiectomy did not seem to add any benefit, combined androgen blockade (addition of anti-androgen) remains superior to LHRH analogue therapy alone [1,2].
The results of the Southwest Oncology Group trial 9346 [3] were reported in early 2013. The study randomized metastatic prostate cancer patients to receive either continuous or intermittent androgen suppression therapy. Of the 3040 patients enrolled, 1535 patients (50.4%) were randomized to either continuous (759) or intermittent therapy (770). The hazard ratio of 1.1 (90% confidence interval 0.99, 1.23) revealed that intermittent therapy was not considered non inferior to continuous androgen deprivation therapy. Median survival was 5.8 years and 5.1 years in the continuous and intermittent arms, respectively. Median OS of extensive disease patients in the intermittent arm was 4.9 years compared with 4.4 years in the continuous arm (HR: 1.02 95% CI (0.85, 1.22)). Median OS of minimal disease patients treated intermittently was 5.4 years as compared with 6.9 years for continuously treated patients (HR: 1.19, 95% CI (0.98, 1.43). 1505 patients who were not randomized due to an inability to achieve a nadir PSA of 4 or less, demonstrated a poor clinical outcome with median survival of 1.7 years.
The recently reported results of ECOG 3805 (CHAARTED) [4] are likely to drastically change our therapeutic sequencing in metastatic prostate cancer. The study evaluated the overall survival benefit of adding docetaxel based chemotherapy to androgen deprivation therapy in metastatic hormone sensitive prostate cancer. The study randomized 790 men with metastatic prostate cancer to receive androgen deprivation therapy +/− 6 cycles of docetaxel chemotherapy. A recent interim analysis of the study revealed that the addition of chemotherapy improved survival outcome, with 3 year OS of 69% with the combination, and 52.5 % with androgen deprivation therapy. Men with extensively metastatic disease (4 or more sites of bone metastases or the presence of liver metastases were the most likely to benefit from the addition of chemotherapy. The results of this randomized trial will lead us to consider early use of systemic therapies to improve life expectancy. A number of other effective therapies are now being tested in the hormone sensitive metastatic setting. SWOG 1216 is a randomized trial of androgen deprivation +/− TAK-700 in metastatic hormone sensitive prostate cancer. A multicenter phase II randomized trial is being activated shortly of LHRH analogue + bicalutamide vs LHRH analogue + enzalutamide in metastatic hormone sensitive prostate cancer.
Therapy of Metastatic CRPC
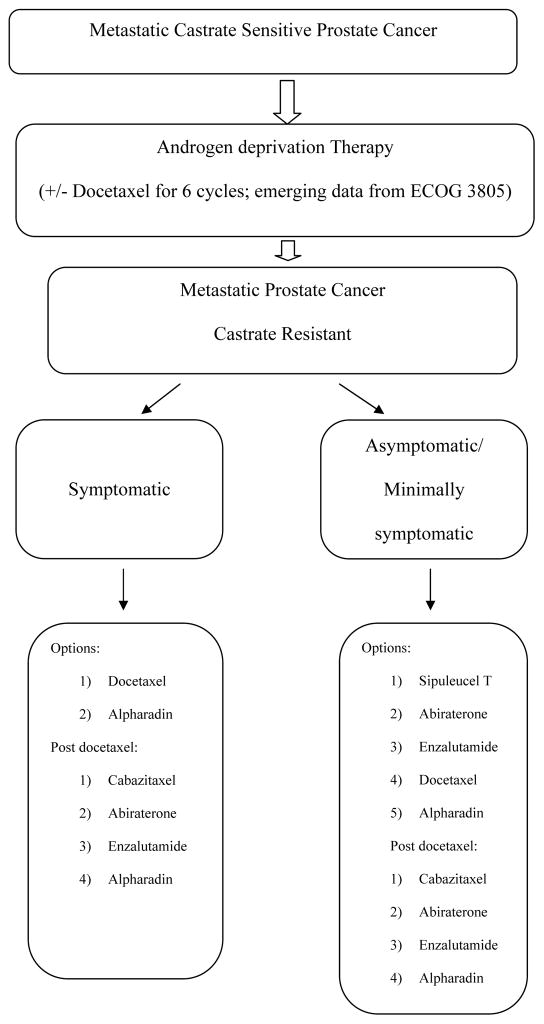
There are multiple therapeutic options available at every juncture of metastatic prostate cancer. At present, all therapies are only approved for CRPC with obvious/ radiologic metastatic disease. To date, no systemic therapy has proven benefit, or received FDA approval, in the setting of PSA only progression in non-metastatic CRPC. The clinical parameters commonly used to determine therapeutic choice are eligibility for chemotherapy and extent of disease related symptoms. Figure 1 illustrates the clinical therapeutic decision making based on current evidence.
Figure 1.
Therapeutic Decision Making in Metastatic Prostate Cancer: The options with randomized trial evidence of OS benefit are mentioned in the flow chart.
Front line therapies in CRPC
Historically, mitoxantrone and steroid combination was the first systemic regimen to demonstrate palliative benefit in metastatic CRPC [5,6]. Despite the lack of survival benefit, this chemotherapy was adopted in the hope of achieving control of disease related morbidities. In 2005, a docetaxel and prednisone regimen, and a combination of estramustine and docetaxel, each demonstrated OS benefit over mitoxantrone and prednisone in independent randomized trials [7,8]. In addition to the OS benefit provided by docetaxel and prednisone, better supportive care, and effective anti-emetic therapies have made the delivery of chemotherapy easier. The acceptance of chemotherapy in advanced CRPC is gradually expanding, and the pendulum is shifting towards earlier therapy. This phenomenon probably resulted in the large difference in OS observed in the mitoxantrone and prednisone control arms between the Kantoff and TAX327 trials (median OS 12.3 months in the study reported by Kantoff et al. and 16.5 months in the TAX 327 trial).
The contemporary trials highlight the outcome differences that have partly resulted from earlier therapy and better supportive care. Both abiraterone and enzalutamide [9, 10] have resulted in a markedly improved radiologic progression free survival (PFS), and a trend towards OS benefit in untreated metastatic castrate resistant prostate cancer [Table 1] [ 7,9, 11–16]. Both these agents attack the androgen-receptor interaction pathway, and demonstrate robust efficacy in advanced prostate cancer. Abiraterone is a CYP-17 inhibitor that suppresses adrenal and tumor microenvironment androgen production, and enzalutamide is a competitive antagonist of the androgen receptor. In a placebo controlled double blind randomized trial, 1088 asymptomatic/minimally symptomatic metastatic CRPC patients were treated with prednisone 5 mg twice daily with or without abiraterone 1000 mg orally daily. Abiraterone therapy doubled the median radiologic PFS to 16.5 months as compared to 8.3 months for patients treated with prednisone alone. OS also improved with hazard ratio of 0.75 (95% CI, 0.61 to 0.93, P = 0.01) leading to the Food and Drug Administration (FDA) approval of abiraterone in the pre-chemotherapy setting of metastatic CRPC [9]. In October 2013, the results of a similar trial comparing enzalutamide versus placebo, were released. The study, consisting of 1715 randomized patients, was halted early by the independent data and safety reporting committee. Enzalutamide therapy resulted in a 30% reduction in the risk of death, (hazard ratio=0.70, p < 0.0001) and 81% reduction in the risk of radiographic progression (hazard ratio=0.19, p < 0.0001). Treatment with enzalutamide resulted in a calculated point estimate for median overall survival of 32.4 months (95% confidence interval, 31.5 months-upper limit not yet reached) versus 30.2 months (95% confidence interval, 28.0 months-upper limit not yet reached) for patients receiving placebo [10]. The promising results and favorable toxicity profiles of both abiraterone and enzalutamide make a strong clinical case for considering either of these medications in the front line treatment of metastatic CRPC. However, the improved outcomes noted above can also be attributed to the stringent patient selection criteria required on both the trials discussed above. The eligibility consisted of asymptomatic to minimally symptomatic patients, with good performance status. It is noteworthy that this patient population even with metastatic CRPC was able to stay on therapy with prednisone alone for a median duration of 8.3 months. Chemotherapy would likely have a similar OS outcome, but with an increased risk of toxicities. It is likely that henceforth the initial therapy of metastatic CRPC is likely to be either enzalutamide, or the combination of abiraterone and prednisone. Clinical trials of sequencing and combinations, with assessment of predictive biomarkers, will help determine therapeutic choices in the future.
Table 1.
FDA approved agents with overall survival advantage in metastatic CRPC.
| Trial [Ref] | Study population | Study arm | Pred use | Size (R) | OS | PFS | PSA RR | Adverse events of interest | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy | ||||||||||
| Tax 327 [7] | CRPC with no prior chemotherapy | 5mg bid | 1006, (1:1:1) | Neutropenia$ | Fatigue | Neuropathy | ||||
| Docetaxel 75mg/m2 Q3wks | 18.9 | NR | 45% | 9.6% | 16% | 9% | ||||
| Docetaxel 30mg/m2 Qwk | 17.4 | NR | 48% | 0.6% | 14.8% | 7.3% | ||||
| Mitoxantrone 12mg/m2 Q3wks | 16.5 | NR | 32% | 6.6% | 10.4% | 2.1% | ||||
| TROPIC [11] | CRPC post docetaxel | 10 mg daily | 755, (1:1) | Neutropenia$ | Fatigue | Diarrhea | ||||
| Cabazitaxel 25mg/m2 Q3wks | 15.1 | 2.8 | 39% | 82% | 37% | 47% | ||||
| Mitoxantrone 12mg/m2 Q3 wks | 12.7 | 1.4 | 18% | 58% | 27% | 11% | ||||
| Hormone Therapy | ||||||||||
| COU-AA-301 [12] | CRPC post docetaxel | 5 mg bid | 1195, (2:1) | Fluid retention | LFT elevation | Hypokalemia | ||||
| Abiraterone 1000 mg daily | 14.8 | 5.6 | 29% | 31% | 10% | 17% | ||||
| Placebo | 10.9 | 3.6 | 6% | 22% | 8% | 8% | ||||
| COU-AA-302 [9] | CRPC pre-docetaxel. mild or no symptoms | 5 mg bid | 1088, (2:1) | Fluid retention | LFT elevation | Hypokalemia | ||||
| Abiraterone 1000 mg daily | NRea | 16.5 | 62% | 28% | 12% | 17% | ||||
| Placebo | 27.2 | 8.3 | 24% | 24% | 5% | 13% | ||||
| AFFIRM, [13] | CRPC post docetaxel | no | 1199, (2:1) | Seizure | Fatigue | Hot Flashes | ||||
| Enzalutamide 160 mg daily | 18.4 | 8.3 | 54% | <1% | 34% | 20% | ||||
| Placebo | 13.6 | 3 | 2% | 0% | 29% | 10% | ||||
| Immunotherapy | ||||||||||
| [14] | Asymptomatic, no visceral mets, most were chemo naïve | No | 127, (2:1) | Rigors | Pyrexia | Tremor | ||||
| Sipuleucel-T 3 infusion Q3wks | 25.9 | 2.7 | NR | 60% | 29% | 10% | ||||
| Placebo | 21.4 | 2.3 | NR | 9% | 2% | 0% | ||||
| IMPACT [15] | No or mild symptoms, mostly Gleason ≦7, most chemo naïve | No | 512, (2:1) | Chills | Pyrexia | Headache | ||||
| Sipuleucel-T 3 infusion Q3wks | 25.8 | 3.7 | 2.6% | 54% | 29% | 16% | ||||
| Placebo | 21.7 | 3.6 | 1.3% | 13% | 14% | 5% | ||||
| Bone Targeted Therapy | ||||||||||
| ALSYMPCA [16] | Symptomatic mCRPC with no visceral met, post docetaxel | NR | 921 (2:1) | Neutropenia$ | Thrombocytopenia$ | |||||
| Rad-223 IV Q4 wks for 6 cycles | 14.9 | NR | NR | 1.8% | 4% | |||||
| Placebo | 11.2 | NR | NR | 0.8% | 2% | |||||
Pred: prednisone, (R): randomization, OS: median overall survival (months), PFS: median progression free survival (months), PSA RR: prostate specific antigen response rate, NR: not reported,
grade 3 or more, NRea: not reached.
Skeletal morbidity has decreased in incidence, and delayed in occurrence, due to bone targeted therapies such as zoledronic acid and denosumab [17,18]. In the future, as more and more effective treatments are developed, the impact of bone targeted therapies is likely to become more blunted. Immunotherapy with sipuleucel T [14,15] has demonstrated OS benefit in patients with asymptomatic/minimally symptomatic metastatic CRPC. Clinical, PSA, or symptom responses are not detected or expected with the therapy and hence patient selection is critical. Studies are ongoing to evaluate biomarkers for patient selection and response assessment. The evidence driven therapeutic sequence in a carefully selected, asymptomatic, slowly progressing, metastatic CRPC patient would be that of sipuleucel T followed by either abiraterone+ prednisone, or enzalutamide [Figure 1].
Combinations of the currently available agents are in clinical trial testing. The Alliance trial of abiraterone +/− enzalutamide in metastatic CRPC will be launched shortly. Numerous trials of combinations with docetaxel have been unsuccessful in demonstrating any OS benefit [19–24]. VEGF targeted therapies, such as with either bevacizumab or aflibercept [19,20], when combined with docetaxel, were associated with greater toxicity and no improvement in survival. Multiple other combinations were tested with either no clinical benefit or even an inferior outcome. These include combinations with GVAX (prostate cancer vaccine), DN-101 (high dose calcitriol), atrasentan (endothelin receptor antagonist), dasatinib and lenalidomide [21–25].
Second line therapies
Treatment strategies can be broadly categorized as chemotherapy, hormonal therapy, immunotherapy and radiation therapy. Table 1 summarizes the FDA approved agents that have demonstrated survival advantage in advanced CRPC. Abiraterone, cabazitaxel and enzalutamide revealed OS benefit in the post docetaxel chemotherapy setting in randomized trials [11–13]. The median OS of the different control arms in each of these trials: prednisone, mitoxantrone chemotherapy, and placebo therapies, were similar. (10.9 months, 12.7 months, and 13.6 months, respectively). The relative OS benefit observed with each of these agents: abiraterone (median survival 14.8 months), cabazitaxel (median survival 15.1 months), and enzalutamide (median survival 18.4 months), as compared to the control arms was also similar. Hence the decision about which agent to use post docetaxel is based on the toxicity profiles of each of the agents, and patient comorbidities. The studies overlapped significantly in that they were conducted in similar patient populations, i.e. in patients pretreated with docetaxel chemotherapy. This results in a paucity of data in a patient population that has been pretreated with docetaxel and either abiraterone or enzalutamide. Future trials in the third line setting will be informative in assessing likelihood of response in a CRPC patient population treated with docetaxel and either enzalutamide or abiraterone.
Radium-223 dichloride (alpharadin), an alpha emitter, selectively targets bone metastases with alpha particles of radiation. Radium-223 [16] was evaluated in a double blind placebo controlled trial in a patient population that was symptomatic from prostate cancer, and either pretreated (30%) or ineligible to receive chemotherapy. Patients were randomized in a 2:1 ratio to receive six injections of radium-223 (at a dose of 50 kBq per kilogram of body weight intravenously) or matched placebo. The primary end point of overall survival favored the use of radium-223 with a hazard ratio of 0.70 and median OS of 14.9 months as compared to 11.2 months in the placebo group. The secondary endpoint of time to the first symptomatic skeletal event was significantly delayed by radium-223 as compared with placebo. (median 15.6 months vs. 9.8 months; hazard ratio, 0.66; 95% CI, 0.52 to 0.83; P<0.001). The tolerability of radium-223, and the demonstrated OS benefit has made this therapy an attractive option in both pre and post docetaxel settings. However, this therapy is active against bone metastases only and patients with significant visceral disease were excluded from the pivotal trial. In addition, the extent of pain control and magnitude of alleviation of other symptoms (besides skeletal events) have not been formally evaluated.
Concurrent palliative and supportive therapy plays a critical role in optimizing systemic therapy in patients with metastatic prostate cancer. Antiemetics and premedications to avert hypersensitivity reactions are some of the key components of care during administration of either docetaxel or cabazitaxel. Close monitoring for cytopenias, dose modifications as necessary for neuropathy related to docetaxel, and for diarrhea and cytopenias related to cabazitaxel, should be an integral part of the chemotherapy care. Primary growth factor prophylaxis is strongly recommended for cabazitaxel chemotherapy and secondary prophylaxis should be considered for either chemotherapy agent if febrile neutropenia occurs. Monitoring for cardiac function, adrenal insufficiency, fluid retention, hypertension and hypokalemia is important in conjunction with abiraterone therapy. Prednisone supplementation is required in combination with abiraterone to alleviate the mineralocorticoid excess and the subsequent toxicities that are related to it. Enzalutamide therapy resulted in a 0.9% risk of seizures. Patients with history of seizures, or any risk factors for seizures, were excluded from enzalutamide therapy. Physician, patient and family awareness and education regarding toxicities enables prompt attention to adverse events and better outcomes with therapy.
Novel Agents with Promising Efficacy in CRPC
Cabozantinib has dual VEGF and c-met inhibition properties that uniquely fit the current need to delay development of resistance and maintain VEGF inhibition in advanced prostate cancer. The agent demonstrates the unique phenomenon of effecting complete responses or normalizations of bone scans in metastatic prostate cancer. In a phase 2 randomized discontinuation study [26], cabozantinib demonstrated broad clinical activity in men with CRPC. An increase in PFS was observed in the cabozantinib arm compared with placebo (median PFS 23.9 vs 5.9 weeks) with a bone scan response seen in 67% of the patients treated.
TAK-700/ Orteronel is an oral, non-steroidal, selective inhibitor of 17,20-lyase, a key enzyme in the production of steroidal hormones [27]. A phase 3 study (C21005) of orteronel plus prednisone compared to placebo plus prednisone in patients with metastatic CRPC following chemotherapy was halted after a pre-specified interim analysis indicated that orteronel plus prednisone was not likely to meet the primary endpoint of improved OS when compared to the control arm (HR 0.894, p=0.226) [28]. The interim analysis did show an advantage for orteronel plus prednisone for the secondary endpoint of radiographic progression-free survival and no toxicity concerns were seen (HR 0.755, p=0.00029). It is likely that the OS benefit noted was blunted due to crossover of the placebo patients to abiraterone therapy which has a similar mechanism of action or to enzalutamide therapy which also affects the androgen-receptor interaction axis, as both these agents had received FDA approval during the trial. Phase III trials are ongoing in hormone sensitive disease (SWOG 1216) and in chemotherapy naïve metastatic CRPC (ELM-PC4).
ARN-509 is a competitive AR inhibitor that is thought to be more potent than enzalutamide [29]. A phase II study showed that ARN-509 has a very high PSA response rate in treatment naïve CRPC (88%) and it appears to retain activity in abiraterone pretreated mCRPC with a PSA response rate of 29%. No seizures were reported. The agent is being evaluated in the setting of non-metastatic CRPC in a double blind placebo controlled randomized trial.
PROSTVAC-VF is a PSA targeted vaccine that was evaluated in a randomized blinded phase II study in 125 men with metastatic CRPC. Men with visceral metastases, cancer-related pain requiring narcotics, prior chemotherapy or PSA >7 were excluded. The primary end point was PFS, which was similar in the PROSTVAC-VF arm and the placebo arm [30]. However, PROSTVAC-VF patients had improved OS over placebo (25.1 vs 16.6 months, HR 0.56, P=.0061). A phase III trial is ongoing with primary endpoint of OS. Immune checkpoint blockade with CTLA-4 inhibition has also demonstrated preclinical efficacy in prostate cancer. Synergistic activity was observed with the combination of radiation therapy and ipilimumab, a CTLA-4 antibody. A phase I/II study [31] revealed clinical activity, and now a randomized clinical trial has been completed and results are awaited.
Clusterin overexpression has been reported to be an important mechanism of chemoresistance in metastatic prostate cancer. OGX-011, an antisense inhibitor of clusterin has demonstrated promising efficacy when added to docetaxel based chemotherapy. A phase II randomized trial revealed an increase in median OS from 16.9 months to 23.8 months with the addition of OGX-011 to docetaxel therapy [32]. Phase III trials of OGX-011 in conjunction with both docetaxel and cabazitaxel are being conducted.
Selected novel agents with promising efficacy are summarized in Table 2 [26–32].
Table 2.
Summary of Selected Novel agents with promising phase II data for the treatment of CRPC
| Drug [Ref] | Mechanism of Action | Dose and schedule | Size | Results | Side effects |
|---|---|---|---|---|---|
| Cabozantinib [26] | Tyrosine kinase inhibitor (MET and VEGFR2 inhibitor). | 100 mg daily with dose adjustment vs placebo in CRPC post chemo | 31 (1:1) | - Median PFS: 23.9 weeks in cabozantinib arm and 5.9 weeks in placebo (HR 0.12; P < .001). | Most common grade 3 adverse events were fatigue (16%), hypertension (12%), and hand-foot syndrome (8%). |
| TAK-700 (Orteronel) [27,28] | CYP17 inhibitor | 300 mg BID, 400 mg BID + prednisone 5 mg BID, 600 mg BID + prednisone, or 600 mg QD | 96 | - PSA response rate: 63%, 52%, 41%, and 62% respectively. - out of 43: 6 PR, 23 SD, 9 PD |
Fatigue (72%), nausea (44%), and constipation (31%). |
| ARN-509 [29] | AR antagonist | 240 mg/day oral. 2 arms reported. A: treatment naïve, B: abiraterone pre-treated. | 46 (1:1) | - PSA response rate: A 88%, B 29%. | Fatigue (30%), abdominal pain (24%), nausea (22%), and diarrhea (17%). |
| PROSTVAC-VF [30] | PSA targeted vaccine | Administered on days 1, 14, 28, 56, 84, 112, and 140. GM-CSF was used. Had a placebo arm. | 125 (2:1) | - Median PFS: 3.8 months in the vaccine arm and 3.7 months in placebo (p=0.6). - Median OS: 25.1 months with vaccine and 16.6 months with placebo (P=0.0061) |
Injection site reactions (12–58%), fatigue (42%), chills (14.6%), pyrexia (18.3%), nausea (20.7%), dizziness (12.2%). |
| Ipilumimab [31] | Anti CTLA-4 monoclonal antibody | 10 mg/kg every 3 wks x 4 doses ± radiotherapy | 50 | - PSA response rate 16%. - CR 2%, SD 12%. |
Diarrhea (54%), colitis (22%), rash (32%), and pruritus (20%) |
| OGX-011 [32] | Clusterin inhibitor | Docetaxel 75mg/m2 and prednisone 5 mg twice daily +/− 640 mg IV weekly of OGX-011 | 82 (1:1) | - PSA response 58% (OGX-011) vs 54% Med PFS 7.3 (OGX-011) vs 6.1 months Median OS 23.8 (OGX-011) vs 16.1 months |
Grade 1–2 infusion reactions, rigors and fevers |
CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease, AR: Androgen receptor, PFS: progressive free survival, OS: overall survival
Conclusions
Figure 1 summarizes the treatment algorithm for metastatic prostate cancer based on current available data. Significant strides have been made with clinically relevant impact on the morbidity and mortality of advanced prostate cancer. The last few years have demonstrated a switch from chemotherapy based regimens to non-chemotherapy options in metastatic CRPC. This has made systemic therapies widely applicable and feasible since even the elderly patients, or those with significant comorbidities can tolerate the treatments. The interspersing and sequencing of the numerous agents now approved for metastatic CRPC requires further study. Development of predictive biomarkers for each of the therapies currently available will reduce costs, enhance outcomes and optimize toxicities.
Summary.
Multifaceted therapeutic paradigms have emerged as new standards in advanced CRPC.
Level 1 evidence from a randomized trial (ECOG 3805) suggests that early consideration of docetaxel based chemotherapy in castration sensitive metastatic disease is indicated.
In addition to chemotherapy, hormone and immune therapies have demonstrated efficacy and present better tolerated systemic therapy options
Novel targets and agents continue to be evaluated in this incurable disease which generally has a terminal prognosis.
Acknowledgments
Sincere thanks extended to Dr Abdel Alqwasmi for his help in manuscript preparation.
Footnotes
Disclosures: Dr Vaishampayan is a speaker for Bayer, Janssen and Astellas/Medivation, consultant for Astellas/Medivation and has research support from Astellas/Medivation.
References
- 1.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 3**.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–1325. doi: 10.1056/NEJMoa1212299. A large well powered study evaluating the strategy of initial hormone therapy in metastatic prostate cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.http://centerformenshealth.com/center-4-mens-health/new-treatment-now-available-that-extends-life-in-men-with-highly-advanced-prostate-cancerThe study results of ECOG 3805 demonstrating OS benefit with early docetaxel chemotherapy may change standard of care in patients with extensive disease metastatic prostate cancer
- 5.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17:2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 8.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 9*.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. Phase III trial demonstrating radiologic PFS benefit with abiraterone and prednisone as compared to placebo and prednisone in metastatic CRPC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MDVN News General release Oct 22, 2013. Medivation and Astellas Announce the Phase 3 PREVAIL Trial of Enzalutamide Meets Both Co-Primary Endpoints of Overall Survival and Radiographic Progression-Free Survival in Chemotherapy-Naive Patients With Advanced Prostate Cancer. * Phase III trial demonstrating radiologic PFS and OS benefit with enzalutamide as compared to placebo in metastatic CRPC.
- 11.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 12.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. Phase III trial demonstrating OS benefit with enzalutamide as compared to placebo in docetaxel pretreated metastatic CRPC. [DOI] [PubMed] [Google Scholar]
- 14.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 15.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16**.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. Phase III trial demonstrating OS benefit with radium-223 as compared to placebo in symptomatic metastatic CRPC either post docetaxel or ineligible to receive docetaxel. [DOI] [PubMed] [Google Scholar]
- 17.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 19.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30:1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tannock IF, Fizazi K, Ivanov S, et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol. 2013;14:760–768. doi: 10.1016/S1470-2045(13)70184-0. [DOI] [PubMed] [Google Scholar]
- 21.Small E, Demkow T, Gerritsen WR, et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC) [abstract]. ASCO - GU Cancers Symposium; 2009. p. Abstract 07. [Google Scholar]
- 22.Scher HI, Jia X, Chi K, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol. 2011;29:2191–2198. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- 23.Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 2013;14:893–900. doi: 10.1016/S1470-2045(13)70294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araujo JC, Trudel GC, Saad F, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14:1307–1316. doi: 10.1016/S1470-2045(13)70479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.http://www.news-medical.net/news/20121012/Lenalidomide-worsens-survival-adverse-events-in-prostate-cancer.aspx.
- 26.Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.http://www.takeda.com/news/2013/20130726_5894.html.
- 28.Patel JC, Maughan BL, Agarwal AM, et al. Emerging molecularly targeted therapies in castration refractory prostate cancer. Prostate Cancer. 2013;2013:981684. doi: 10.1155/2013/981684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rathkopf DE, Morris MJ, Fox JJ, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31:3525–3530. doi: 10.1200/JCO.2013.50.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi KN, Hotte SJ, Yu EY, et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(27):4247–4254. doi: 10.1200/JCO.2009.26.8771. [DOI] [PubMed] [Google Scholar]