Abstract
This paper examines the interaction between social control and social risk mechanisms and genes within the dopaminergic system (DAT1 and DRD2) as related to serious and violent forms of delinquent behavior among adolescents and young adults. We use nine waves of data from the National Youth Survey Family Study to examine the relevance of protective or risky social factors at four social levels including school, neighborhood, friends, and family within the gene-environment interaction framework. We extend previous work in this area by providing a testable typology of gene-environment interactions derived from current theories in this area. We find consistent evidence that the associations between putatively risky genotypes and delinquent behavior are suppressed within protective social environments. We also provide some evidence that supports the differential susceptibility hypothesis for these outcomes. Our findings largely confirm the conclusions of previous work and continue to highlight the critical role of the social environment within candidate gene studies of complex behaviors.
Introduction
In the wake of decades of research, there is consensus among social scientists that variation in nearly all behavioral traits is the product of both genetic and environmental factors (Ferguson, 2010; Rhee & Waldman, 2002; Turkheimer, 2000). The strongest evidence for this perspective is that heritability estimates for most traits vary considerably across environments (Moffitt, 2005). Stated differently, genetic influences on a given trait can—and often do—depend on forces in the environment, a phenomena referred to as gene-environment interaction (GxE) (Rutter, 2006). While variation in heritability estimates capture the latent influences of genes, scholars have recently focused their attention on uncovering the specific genes that might interact with measured environments to predict various phenotypic outcomes. Along these lines, a landmark achievement occurred over a decade ago when Caspi et al. (2002) reported the most widely cited measured GxE in the prediction of violent and antisocial behavior.
In the wake of the Caspi et al. (2002) study, researchers have begun to examine the relevance of the gene-environment interplay more widely, with growing interest aimed at further illuminating the contribution of GxEs as sources of variance in delinquent behavior (Beaver, DeLisi, Wright, Vaughn, 2009; Guo, Roettger, & Cai, 2008; Simons et al., 2011). Emergent findings in this area suggest that an individual’s likelihood of engaging in delinquent behavior as a result of environmental triggers might increase depending upon their genes. Because delinquent behavior is a highly polygenic trait, it stands to reason that single genes confer only a minor increase in the odds of committing a given delinquent act (Plomin et al., 2008). Despite exerting rather small main effects, the influence of certain genotypes may become magnified when coupled with risky environments (or vice versa). These general associations (GxEs) continue to be demonstrated in the literature with increasing frequency in a diverse range of samples (Caspi et al., 2002; Freese & Shostak, 2009; Guo et al., 2008; Kim-Cohen et al., 2006; Moffitt, 2009; Simons et al., 2011; Taylor & Kim-Cohen, 2007). However, there is also evidence that some of the most “established” GxE associations do not replicate across independent samples. Specifically, Risch et al. (2009) examine the link between 5HTTLPR genotype and depression as a function of stressful life events similar to those reported in Caspi et al. (2003) using 14 independent samples and they do not find evidence for a GxE association in this well powered (n=14,250) meta-analysis. As such, it is critical to evaluate previously published GxE associations with new sources of data and to expand upon this previous work with additional phenotypes and environmental moderators.
In this paper, we use data from nine waves of the National Youth Survey Family Survey (NYSFS) to examine gene-environment interactions in the prediction of antisocial behavior. We examine whether the specific alleles within two genes in the dopaminergic pathway (DRD2 and DAT1) interact with neighborhood, familial, school, and peer factors to predict serious and violent delinquency during adolescence and young adulthood. The NYSFS provides a rich set of repeated measures across multiple social domains from a national sample of respondents. Most importantly, we also elaborate on previous research (see Guo et al., 2008) by providing a testable typology of gene-environment interactions derived from existing theory that help to frame the results of this and other papers in this area.
Gene-environment interaction: A brief overview
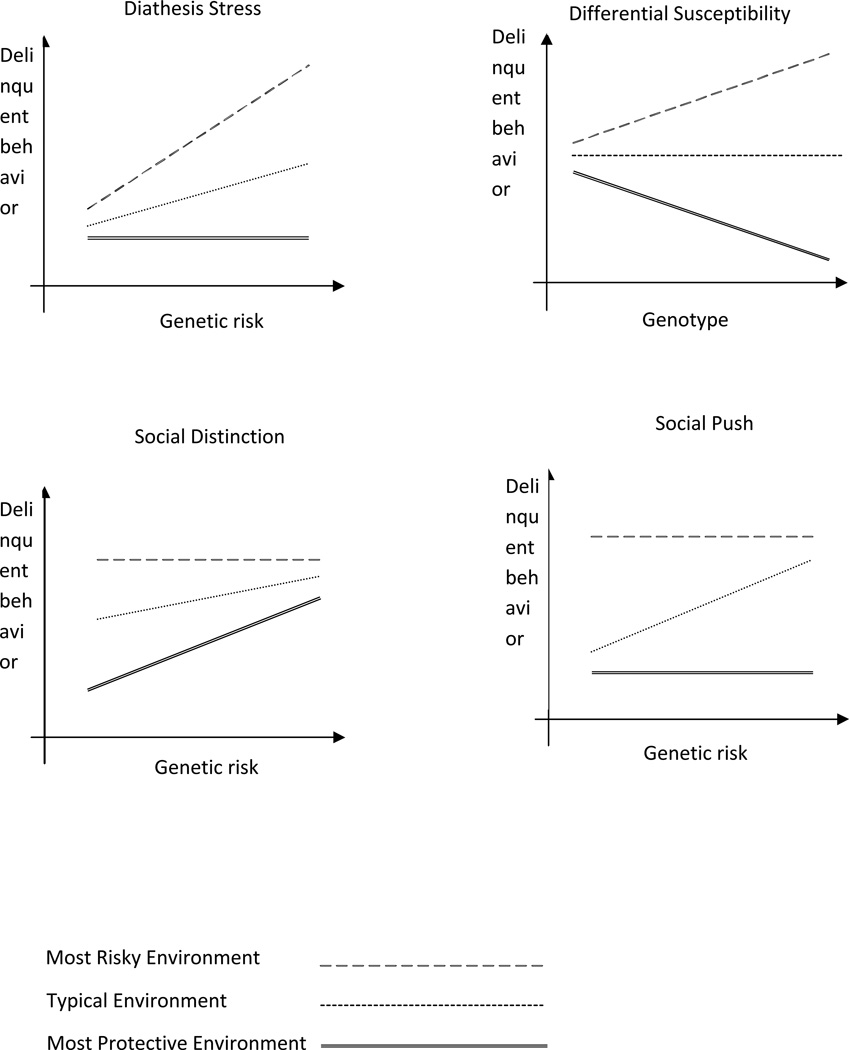
The growing body of GxE scholarship (Shanahan & Hofer, 2005; Shanahan & Boardman, 2009) has outlined four distinct ways in which genes and the environment might coalesce non-additively to influence delinquent phenotypes: 1) diathesis-stress, 2) differential susceptibility, 3) social push, and 4) social distinction. Each model is described graphically in Figure 1. The diathesis-stress hypothesis suggests that unobserved genetic factors may predict delinquent behaviors for individuals who encounter adverse environments of some variety. In this regard, risky social contexts may be required to trigger genetic tendencies for adverse behaviors (Shanahan & Hofer, 2005) and it represents the primary GxE model within the biological and social sciences (Reiss & Leve, 2007). According to this perspective, environmental triggers remain the fundamental cause (Link & Phelan, 1995). The social control model (Shanahan & Hofer, 2005) is statistically indistinguishable from the diathesis-stress model but it emphasizes the presence of social resources rather than exposure to stress. In other words, social control mechanisms reduce genetic risks and social risks exacerbate genetic risks. As such, the difference between the social control model and the diathesis stress model is simply the focus on the healthy or unhealthy end of the environmental spectrum. To illustrate, previous research suggests that residing in a state with high levels of taxes on cigarettes reduces the relative influence of genes on the likelihood that an individual will become a regular smoker (Boardman, 2009) which is taken as a form of social control.
Figure 1.
Conceptual gene-environment interaction models.
Second, Belsky & Pluess (2009) have argued that the explicit focus on risky environments overlooks the possibility that particular individuals are more susceptible than the typical person to the influences of their social environments in both a “for better and for worse” fashion (for a recent review of this perspective see Ellis, Boyce, Belsky, Bakermans-Kranenburg, Van Ijzendoorn, [2011]). As shown in Figure 1, not only will susceptible individuals experience the worst outcomes in the worst environments but they will also have the best outcomes in the best environments (Belsky & Beaver, 2011). Simons and his colleagues (2011), for instance, demonstrated that carriers of “plasticity alleles” (e.g., the 7R allele in DRD4 and the ‘short’ allele in 5HTTLPR) were significantly more likely to exhibit signs of aggression when they are in the most adverse environments and significantly more likely to have the lowest symptoms of aggression when they are in the most favorable social environments. This is precisely the anticipated association of the differential susceptibility model and their work highlights the need to consider the full range of social environments rather than just risky vs. non-risky social contexts because this model anticipates no association between genotype and delinquent behavior for those in typical environments.
The third model has been called the social distinction model and it predicts a very different association than the diathesis stress model. The key difference of this model is that it does not necessarily anticipate a causal gene-environment interaction; the environment isn’t ‘causing’ genes to operate differently. Rather, environmental variation in more risky contexts makes it difficult to identify genetic associations. As such, it is within the most protective environments in which genotype becomes the most salient. For example, Boardman, Barnes, Wilson, Evans, & Mendes de Leon (2012) use data from the Chicago Health and Aging Project to examine the link between the e4 allele in the APOE gene and cognitive decline among older adults in Chicago. They show that the risky allele is only weakly associated with change in cognitive functioning for residents of the most disorganized neighborhoods (e.g., those with trash in the streets, the presence of vandalism, poorly maintained sidewalks, etc.). The effect becomes much more pronounced, however, for residents in the most stable, organized, and ordered neighborhoods.
The last interaction model in Figure 1 has been called the social push model (Raine, 2002) and it emphasizes the difference between normal and atypical social environments rather than socially stressful environments, per se. This model hypothesizes that genetic factors are the most salient within the average or typical environments; on either extreme the social environment is pushing the phenotype and genotype does less to distinguish among individuals. Boardman et al. (2012b) have shown this with social factors related to obesity. They show that the heritability of BMI is the highest among students who attend schools with typical norms about body size but is significantly reduced in schools that have strong norms about very high or very low levels of BMI because the environment is driving BMI in those extreme contexts and genotype has little room to differentiate among individuals.
To examine the relevance of these gene-environment interaction models we focus the current study on two genes in the dopaminergic system that have been linked to delinquent behavior in previous research (Beaver, Wright, DeLisi, Daigle, Swatt & Gibson, 2007; Guo et al., 2008): DRD2 and DAT1. DRD2 is a gene that encodes the dopamine D2 receptor and it plays a fundamental role in the complex circuitry of the human brain. Variations within DRD2 are associated with ADHD, impulsivity, antisocial behavior and addiction (Colzato, van den Wildenberg, Van der Does, & Hommel, 2010; Guo, Roettger, & Shih, 2007; Groman & Jentsch, 2011; Marino et al., 2004). The Taq1A locus (rs1800497) is 9.4kb downstream from the DRD2 gene which is located on chromosome 11 (11q23). Individuals who possess the A1 allele are found to be at higher risk for behavioral issues relative those with the A2 variant of the gene and previous research has shown that the A1 allele is not related to delinquency for those with higher levels of closeness within their families (Guo et al., 2008). Vaske, Wright, & Beaver (2011) show that increasing number of A1 alleles in DRD2 is significantly associated with delinquency but only among those who had been victims of crimes themselves. Specifically, only those who had recently reported being shot at, stabbed, jumped, or had someone pull a knife or gun on them demonstrated an increased risk of delinquency as a function of their DRD2 genotype. These results support the diathesis-stress perspective. DAT1 is a gene which is involved in the regulation of the neurotransmitter dopamine in the central nervous system where individuals with the 10R variant of this gene are found to have an increased risk for a number of behavioral issues, including antisocial behavior, ADHD, and substance abuse (Cornish et al., 2005; Guo, Cai, Guo, Wang, & Harris, 2010). We examine the number of copies of the 10R variant of DAT1 in GxE analyses. Evidence for the social distinction GxE perspective with respect to the 10R allele in DAT1 has been shown by Vaughn, DeLisi, Beaver, & Wright (2009) who find that the 10R allele is significantly associated with the number of police contacts among adolescents who interact with the least number of delinquent peers.
In sum, we focus on these two polymorphisms because they are implicated in same neurological pathway (the dopaminergic system), they are both fairly common in the population, both polymorphisms have shown main effects for delinquent behavior (Guo et al., 2007), and both have shown interactive effects with various aspects of the environment (Guo et al., 2008, Simons et al., 2011; Vaske et al., 2011).
Methods
Data
We use data from the National Youth Survey and Family Study (NYSFS), a prospective longitudinal study of 1,725 respondents from adolescence through adulthood. The NYSFS is based on a nationally representative multi-stage probability sample of households in the continental United States (Elliot, Huizinga, & Menard, 1989). Originally selected in 1976, respondents were between the ages of 12–17 and characterize a multi-cohort sequential longitudinal design. Annual interviews were conducted between 1977 and 1981 and in three-year intervals from 1984 to 1993. In 2002 and 2003, follow-up interviews were conducted (age range: 37–44) during which additional behavioral and DNA data were collected from the original respondents. Data collection was conducted primarily through confidential, in-person interviews, though some interviews at later waves were conducted by telephone. Genomic DNA data were voluntarily collected from 80% of respondents who provided buccal (cheek) swab DNA samples pre-amplified using a well-established method (Zhang et al., 1992). Data obtained using this DNA are high-quality and these methods are reliable for genotyping (Anchordoquy, McGeary, Liu, Krauter, & Smolen A, 2003; Haberstick & Smolen, 2004). Of the 1,725 original respondents at Wave 1, 1,677 (97% of the original sample) survived and were eligible for interviews at Wave 10. At wave 10, 1,266 (73% of the original sample) were interviewed and 988 (57% of the original sample and 78% of those interviewed) provided DNA. After eliminating individuals with poor DNA quality and those with missing values on the key dependent variables, our final sample includes 724 respondents.
Measures
Dependent variables
Serious delinquency is measured with an eleven item scale of self-reports for items that could lead to arrest and incarceration. Overall, we use eleven items which were asked in a consistent manner over a period of nine waves. These questions were the number times a respondent engaged in the following acts during the prior twelve months: (1) attacking someone; (2) deliberately damaging another person’s property; (3) using force to obtain things from others; (4) carrying a hidden weapon; (5) stolen anything worth less than $5; (6) stolen anything worth between $5-$50; (7) stolen anything worth more than $50; (8) sold marijuana; (9) sold hard drugs; (10) hit a parent; and (11) had forced sex against another’s will. Frequencies were collapsed such that ‘0’ =occurred zero times, ‘1’ =occurred once or twice, ‘2’=occurred three or four times, and ‘3’=occurred five or more times. At each wave, scores from self-reports were summed together to create a general score for serious delinquency. Violent delinquency is the total number of times an individual reported attacking someone and having forced sex against another’s will. Table 1 presents descriptive statistics for the dependent variables, plus age, across the nine waves of the NYSFS used in the present study.
Table 1.
Serious and violent delinquency across the lifecourse: National Youth Survey, Waves 1–9.
| Wave | Serious (ln) | Violent (ln) | Age |
|---|---|---|---|
| 1 | .49 | .11 | 13.77 |
| (.75) | (.38) | (1.96) | |
| 2 | .43 | .10 | 14.77 |
| (.72) | (.45) | (1.96) | |
| 3 | .46 | .08 | 15.77 |
| (.73) | (.38) | (1.96) | |
| 4 | .43 | .17 | 16.77 |
| (.75) | (.60) | (1.96) | |
| 5 | .42 | .19 | 17.77 |
| (.72) | (.66) | (1.96) | |
| 6 | .36 | .16 | 20.86 |
| (.67) | (.58) | (1.98) | |
| 7 | .33 | .28 | 24.22 |
| (.63) | (.77) | (2.04) | |
| 8 | .25 | .15 | 26.96 |
| (.55) | (.57) | (2.02) | |
| 9 | .24 | .24 | 30.17 |
| (.54) | (.75) | (2.05) | |
| Total | .38 | .17 | 19.96 |
| (.68) | (.59) | (5.78) | |
Note: Data come from 9 waves of the National Youth Survey Family Study (NYSFS). N = 724. Cell entries represent means and standard deviations (in parantheses).
Protective and risk factors
We emphasize four protective and risk factors that are multilevel (e.g., families, schools, peers, and neighborhoods) and multidimensional (e.g., social control and social risk). All environmental measures are assessed at Wave 1 of the study. Family closeness is a self-report measure that includes five summed items and asks whether: respondent feels like an outsider (reverse coded), family listens if respondent has a problem, respondent is lonely when with family (reverse coded), respondent feels close to family, and family does not take interest in problems (reverse coded). Each item ranged from 1 (strongly disagree) to 5 (strongly agree; α =.72).
School attachment is a self-report measure that includes 6 reverse-coded and summed items ranging from 1 (strongly disagree) to 5 (strongly agree) and asks the extent to which: a respondent is not often asked to take part in school activities, a respondent is not called on, a respondent feels that nobody at school cares, a respondent does not feel as if he or she belongs at school, a respondent often feels lonely at school, and teachers do not ask the respondent to work on projects (α =.65).
Peer delinquency is a self-report measure that includes 6 summed items, asking how many friends had: cheated on school tests, damaged property, used marijuana, stole something worth less than $5 and more than $50, hit or threatened to hit, used alcohol, broke into vehicles or buildings to steal, sold drugs, and suggested that respondent break the law. Each item ranged from 1 (none of them) to 5 (all of them; α =.75).
Neighborhood disorganization is a parent-report measure that includes six items, summed together, asking whether the following problems occurred in the parent’s (and respondent’s) neighborhood: vandalism or buildings broken into, winos and junkies, abandoned houses, burglaries and thefts, run down and poorly kept buildings, or assaults and muggings. Each response ranged from 1 (not a problem), 2 (somewhat a problem), or 3 (a big problem) (α=.77).
Control variables
All 5 of the following control variables were measured at baseline, Wave 1. Parent-reported control variables include parent unemployed which is coded 1 if the principal wage earner is unemployed and 0 if otherwise. In addition, parental poverty is coded 1 if the family received any public assistance including food stamps or welfare payments during the last year and 0 if otherwise. Parent attended college is coded 1 if the parent respondent attended college and 0 if otherwise. Respondent’s household size is a continuous measure indicating the number of persons currently residing in the household including family and non-family members. Self-reported grades from the first four waves of data are used to create a continuous measure of grade point average (GPA). We also control for respondent’s age (and age squared) at each wave of interview (Wave 1 through Wave 9), and race (two groups, with minority respondents consisting of those classified as Black, Hispanic, Native American, Asian, or other race/ethnicity and with White as the reference group).
Analytic Strategy
We nest wave-specific observations within individuals using a simple multi-level model (random intercept). We use the xtmixed package in STATA 12.0 to estimate all models. As described elsewhere, Gelman & Hill (2007), these models allow us to describe the delinquency of individual j at time i as follows:
| [1] |
| [2] |
In this specification, the intercept (aj)captures between person variation in delinquency indicated by and describes the within person error variance. All models adjust for the control variables described above and we include an interaction between each environmental variable and each genetic polymorphism. In order to examine the different GxE models described above, we distinguish between three levels of risk (or protection) in order to have a low risk, typical or average risk, and high risk. If we do not observe an association between genotype and delinquency in the most protective environments but we do in the riskier environments, then our findings would support the social trigger model. Again, these models are detailed in Figure 1. The most important aspect of this categorization is the fact that we will have an “average” or “typical” environment that is characterized by the center of the distribution. If the social push perspective is accurate, then we should see the largest genetic associations among those with the average level of risk exposure.
Results
Tables 3 and 4 present the results from 16 multilevel (random intercept) regression models. For parsimony, only the main genetic, main environment, and gene-by-environment interaction terms for each regression is presented in the table (full results available upon request). Of the 16 models, we find evidence of significant environmental moderation (p<.05) in seven cases which is far above the number that we would observe due to chance alone. Most importantly, six of these seven interactions occurred with protective forms of social control as opposed to social risks. Specifically, three of the four models for family closeness and three of the four models for school attachment indicate that the presence of social resources reduces the influence of genotype on both serious and violent forms of delinquency and this is consistent for both DRD2 and DAT1. Evidence for the social trigger or diathesis stress model only appears in one of the 16 models. In this case, DAT1 is not associated with violent delinquency for those who reside in the least risky neighborhoods but this polymorphism significantly predicts violent behaviors for those in the most disorganized communities.
Table 3.
DRD2 Genotype, Delinquency, and Gene-Environment Interactions
| Family Closeness |
Peer Delinquency |
Neighborhood Disorganization |
School Attachment |
|
|---|---|---|---|---|
|
Serious Delinquency |
||||
| Environment [Low] | ||||
| Average | 0.01 | 0.14** | 0.01 | −0.03 |
| (0.05) | (0.05) | (0.04) | (0.05) | |
| High | −0.03 | 0.38*** | 0.04 | −0.04 |
| (0.05) | (0.00) | (0.06) | (0.05) | |
| DRD2 Genotype | 0.13* | −0.01 | −0.04 | 0.04 |
| (0.05) | (0.05) | (0.04) | (0.05) | |
| GxE Estimates | ||||
| DRD2* Average | −0.17* | −0.04 | 0.05 | −0.06 |
| (0.07) | (0.07) | (0.06) | (0.06) | |
| DRD2* High | −0.21** | 0.08 | 0.05 | −0.11 |
| (0.05) | (0.07) | (0.08) | (0.07) | |
| Violent Delinquency | ||||
| Environment [Low] | ||||
| Average | −0.04 | 0.03 | −0.02 | −0.02 |
| (0.03) | (0.03) | (0.02) | (0.02) | |
| High | −0.05* | 0.06* | 0.02 | 0.01 |
| (0.02) | (0.03) | (0.03) | (0.02) | |
| DRD2 Genotype | 0.06* | 0.00 | −0.01 | 0.05* |
| (0.02) | (0.03) | (0.02) | (0.02) | |
| GxE Estimates | ||||
| DRD2* Average | −0.07* | −0.01 | 0.02 | −0.05+ |
| (0.03) | (0.03) | (0.03) | (0.03) | |
| DRD2* High | −0.08* | 0.02 | 0.04 | −0.07* |
| (0.03) | (0.03) | (0.04) | (0.03) | |
Note: Cell entries represent coefficients from a multilevel model in which observations are nested within individuals across 9 waves of data from the NYS (n=724). Standard errors are provided in parentheses below. All models control for gender, age, age squared, race/ethnicity, parental education, parental employment status, parental poverty status, family size, and grade point average. Boxed estimates are presented graphically in Figure 2.
p < 0.10
p < 0.05
p < 0.01
p < 0.001
Table 4.
DAT1 Genotype, Delinquency, and Gene-Environment Interactions
| Family Closeness |
Peer Delinquency |
Neighborhood Disorganization |
School Attachment |
|
|---|---|---|---|---|
| Environment [Low] | Serious Delinquency | |||
| Average | 0.15 | 0.16 | −0.15 | −0.01 |
| (0.11) | (0.11) | (0.09) | (0.10) | |
| High | 0.10 | 0.35** | −0.16 | 0.20+ |
| (0.11) | (0.11) | (0.12) | (0.11) | |
| DAT1 Genotype | 0.12* | 0.03 | −0.05 | 0.07 |
| (0.05) | (0.05) | (0.04) | (0.05) | |
| GxE Estimates | ||||
| DAT1* Average | −0.15* | −0.03 | 0.11* | −0.02 |
| (0.07) | (0.07) | (0.05) | (0.06) | |
| DAT1* High | −0.14* | 0.03 | 0.15* | −0.18** |
| (0.06) | (0.07) | (0.07) | (0.06) | |
| Violent Delinquency | ||||
| Environment [Low] | ||||
| Average | −0.02 | 0.01 | −0.07 | 0.02 |
| (0.05) | (0.06) | (0.04) | (0.05) | |
| High | −0.05 | 0.09 | 0.03 | 0.11* |
| (0.05) | (0.05) | (0.59) | (0.05) | |
| DAT1 Genotype | 0.02 | 0.01 | −0.01 | 0.04 |
| (0.02) | (0.03) | (0.02) | (0.02) | |
| GxE Estimates | ||||
| DAT1* Average | −0.04 | 0.01 | 0.04 | −0.04 |
| (0.03) | (0.04) | (0.03) | (0.03) | |
| DAT1* High | −0.02 | −0.01 | 0.00 | −0.07* |
| (0.03) | (0.03) | (0.04) | (0.03) | |
Note: Cell entries represent coefficients from a multilevel model in which observations are nested within individuals across 9 waves of data from the NYS (n=724). Standard errors are provided in parentheses below. All models control for gender, age, age squared, race/ethnicity, parental education, parental employment status, parental poverty status, family size, and grade point average. Boxed estimates are presented graphically in Figure 2.
p < 0.10
p < 0.05
p < 0.01
p < 0.001
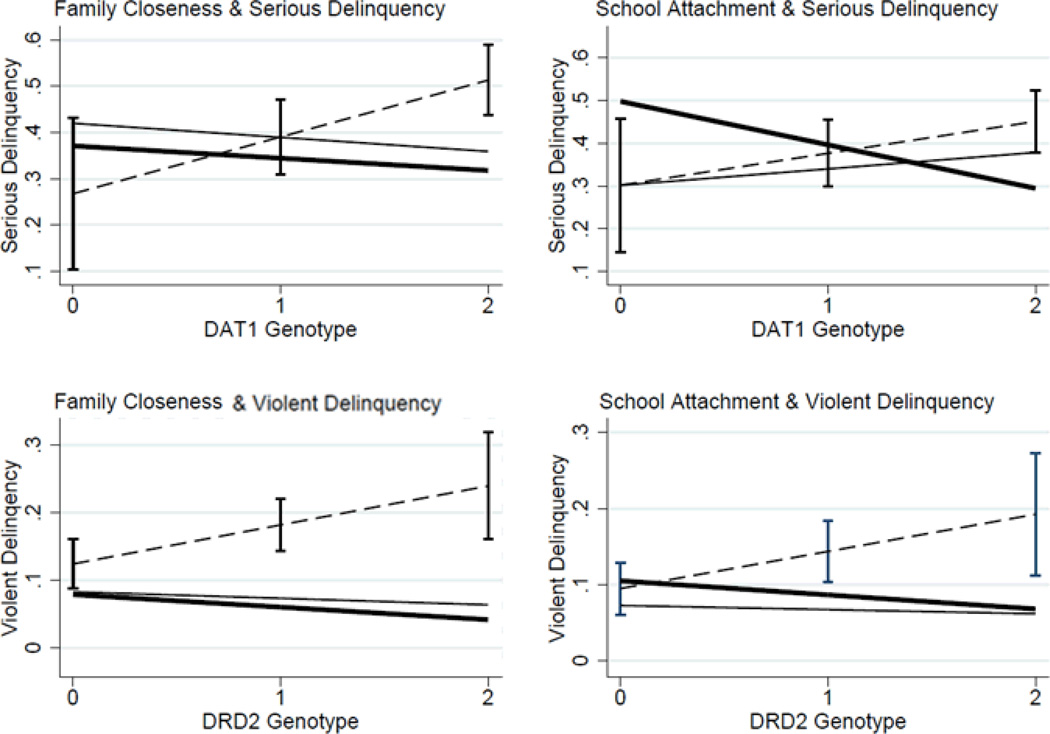
To better differentiate social control (as a protective mechanism) compared to the other GxE models, we plot the fitted values from four of the regression models using the margins command in STATA 12.0. We focus on family closeness and school attachment because as shown in Tables 3 and 4, these environmental factors demonstrate the most consistent evidence for GxE associations. These interactions are shown in Figure 2. The dashed line represents the estimated level of delinquency as a function of genotype for those with the lowest level of social resources. For the top left panel, this line represents those with the lowest level of reported family closeness. The solid heavy line is for those with the highest level of family closeness (or school attachment) and the lighter solid line is the average. Several important relationships are evident in this figure. First, in all four cases, genotype for both genes in the dopaminergic system is only associated with an increased risk of both forms of delinquent behavior for those without attachment to family or to school. In other words, the level of control is less relevant than the absence or presence of control mechanisms. This is important theoretically because it suggests that for most individuals (those who are exposed to average and high levels of social control mechanisms), genotype is not significantly linked to either form of delinquency (as evidenced by the flat lines). Second, there is no evidence to support the social push or social distinction models. In no case is genotype for those from the most supportive environments (the dark solid black lines) positively associated with delinquency (e.g., no support for social distinction) and no evidence to indicate that genotype is only linked to delinquent behavior in the average environments (e.g., no support for the social push model). In the case of school attachment, DAT1, and serious delinquency (upper right corner of Figure 1), we present evidence in support of the differential susceptibility model. Specifically, the weakest genetic association is for those in the average level of school attachment but most importantly, DAT1 genotype confers a risk for those in the riskiest environments (those with low school attachment) and a protective factor among those with the strongest attachment to their schools.
Figure 2. Gene-environment interactions: family closeness, school attachment, dopamine-related genotypes and serious and violent delinquency.
Note: Estimates derived from Tables 3 and 4 using the margins command in STATA 12.0. The dashed line is the lowest level of social control (or highest social risk) and the thick solid line is the highest social control (lowest risk). Confidence intervals are provided for the high risk group to indicate the significance of the observed difference between high risk and the other groups.
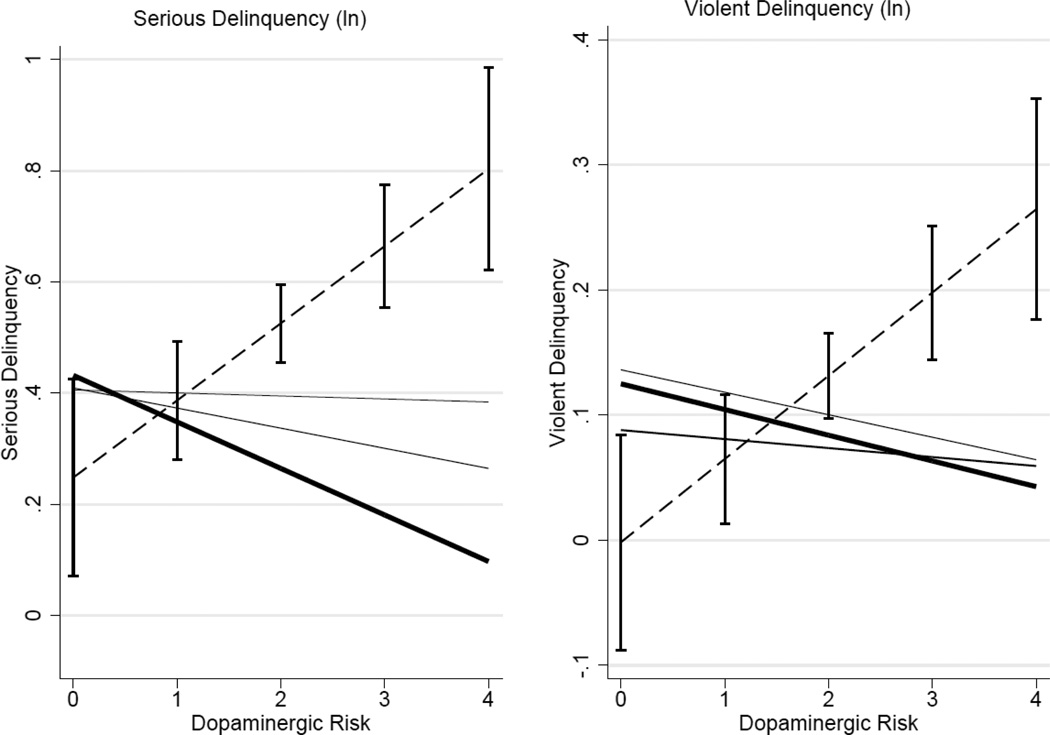
To further explore the nature of the GxE associations presented above, we created a cumulative measure of dopaminergic risk (sum of genotype scores for DRD2 and DAT1) and environmental risk (total number of domains in which individuals have the lowest level of support or the highest level of risk). As shown in the previous figure, most of the environmental moderation seems to be in the extreme cases in which persons have the lowest level of family closeness and school attachment. We took the extreme values for each of the four measures of the environment and summed these together. The regression results are presented in Table 5 and they are graphically presented in Figure 3. The dashed line indicates those that have the riskiest level of school attachment, neighborhood disorganization, peer delinquency, and family attachment. And the solid black line is those that have one or none of these risks. Again, we show that the strongest link between genotype and both forms of delinquency is among those with no forms of social control available to them. There is very little genetic association for those in the average levels for both outcomes, but in the case of serious delinquency, there is additional support for the idea that those in the most integrated, safe, and supported contexts demonstrate a strong and negative association between their dopaminergic profile and their risk of engaging in serious delinquency. This cross over association is in line with the differential susceptibility model and it indicates that some people do quite well in the best environments and incredibly poorly in the worst and that this characteristic (vulnerability) may have its source in genes related to the dopaminergic system (Simons et al., 2011). The differential susceptibility model is important for social and genetic epidemiologists because it provides yet another physiological mechanism through which the environment gets “under the skin” via socially sanctioned behaviors.
Table 5.
Cumulative environmental by cumulative dopaminergic risk for serious and violent forms of delinquency.
| Serious (ln) | Violent (ln) | |
|---|---|---|
| Environmental risk [Ref = 0] | ||
| 1 | 0.16 | 0.14* |
| (0.11) | (0.05) | |
| 2 | 0.16 | 0.09 |
| (0.11) | (0.06) | |
| 3 | 0.18 | 0.13* |
| (0.13) | (0.06) | |
| Dopaminergic Risk (G) | 0.14*** | 0.07** |
| (0.04) | (0.02) | |
| GxE [Env Risk = 0] | ||
| 1 | −0.14** | −0.08** |
| (0.05) | (0.03) | |
| 2 | −0.18** | −0.07** |
| (0.05) | (0.03) | |
| 3 | −0.22*** | −0.09** |
| (0.06) | (0.03) | |
Note: Cell entries represent coefficients from a multilevel model in which observations are nested within individuals across 9 waves of data from the NYS (n=724). Standard errors are provided in parentheses below. All models control for gender, age, age squared, race/ethnicity, parental education, parental employment status, parental poverty status, family size, and grade point average.
p < 0.10
p < 0.05
p < 0.01
p < 0.001
Figure 3. Cumulative genetic and environmental risk for serious and violent forms of delinquency.
Note: Estimates derived from Table 5 using the margins command in STATA 12.0. The dashed line is the lowest level of social control (n=152) and the thick solid line is the highest social control (n=138). Confidence intervals are provided for the high risk group to indicate the significance of the observed difference between high risk and the other groups. In this case, risk is the cumulative number of risky environments. The dashed line describes the estimated effect of increasing number of dopaminergic alleles for those with no social resources from friends, family, neighborhoods, and schools. The solid black line is those who have resources in at least 3 of these domains.
Discussion
Summary
The objectives of this study were two fold—to build upon previous research in this area and to examine a typology of gene-environment interactions. As such, our paper frames the emerging empirical GxE results using the latest gene-environment interaction theory (Belsky & Pluess, 2009; Ellis et al., 2011). We also highlight the need for researchers to consider differences among individuals regarding their likelihood of responding to a particular environmental factor and in which direction they may respond. Inquiry into the interaction between genetic characteristics of individuals and the social, physical, and cultural characteristics of their local environments has grown considerably in the past decade (Freese & Shostak, 2009; Boardman et al., 2010; Simons et al., 2011). Efforts to distill a generalizable typology of gene-environment interaction effects (Shanahan & Hofer, 2005) has made significant progress and we are at a point where we can summarize the patterns of main and interactive effects to gauge the utility of the models within the existing GxE paradigm. To date, however, very little efforts have been made to synthesize this large and diverse body of work in a way that allows us to know the specific phenotypes, environments, genotypes, and the timing of gene-environment interactions that are the most relevant for understanding who, how, and when certain individuals are at the greatest risk of engaging in delinquent behaviors.
In large part, our results support previous work that makes the following point: to understand the extent to which genetic polymorphisms are associated with complex behaviors like delinquency, it is critical that people and their genes are properly contextualized. Absent a full accounting of the environments in which delinquent behaviors are initiated, it is very difficult to make a general statement about the “effect” of any specific polymorphism. We examined four alternative GxE models and the bulk of our results tend to support the social control version of the stress-diathesis model. Conceptually the social control and diathesis-stress models are quite similar but the weaker moderating role played by stressful social environments compared to the strong and consistent role played by controlled and supportive social environments suggests that this specific phenotype (e.g., delinquent behavior) is best understood with the social control model. In other words, the bulk of our results suggest that some genetic effects may be less pronounced when individuals are raised, socialized, and are educated in the most stable and healthy social environments. Importantly, while these models generally support the social control perspective, there is some tentative evidence for the differential susceptibility hypothesis with respect to DRD2 and serious delinquency. Carriers of two copies of the A1 allele at DRD2 are significantly more likely than those with one or no copies of the A1 allele to engage in serious delinquency when there is a low level of family closeness but they are significantly less likely than those with no copies of the A1 allele to report higher delinquency when they perceive a high level of family closeness.
One of the main contributions of our paper is importance of considering the full range of environments when examining different models in the GxE typology. Most GxE studies use a binary indicator or a continuous measure of environmental exposure which makes it impossible to evaluate the relevance of the social push model. Further, the ordinal approach that we used provides information about the functional form of environmental moderation. Importantly, this approach led us to conclude that it is the most risky environments in which genotype emerges as a factor related to problem behavior. This is the strong version of the social trigger (Shanahan & Hofer, 2005) model which is somewhat different than the additive model provided by Caspi et al. (2002).
Additional Considerations
Although our research adds to the existing GxE literature, there are several points that that readers should consider when interpreting the results of our study. First, previous research has shown that the composition of NYS participants changes over time such that the later waves of the study may not be generalizable to the entire population (Brame & Paternoster, 2003). Specifically, the most likely to offend are also more likely to drop out of the study. As such, our persistent offending group may be comprised of an increasingly select group of offenders for reasons that are different from the social and genetic moderators that we present. Second, as discussed above there is growing skepticism in the social and behavioral sciences about the interpretation of p-values from GxE estimates as evidence for ‘significant associations.’ Specifically, Duncan & Keller (2011) make a strong case that most candidate gene by environment interaction results are likely false positives. One would have to make unreasonable assumptions about the magnitude of the main genetic effects and magnitude of the environmental moderation to have a distribution of GxE estimates found in the literature and they argue that most studies are grossly underpowered to detect the associations. In ancillary analyses (results available upon request), we simulated data based upon the effect sizes and covariance estimates across environmental levels using distributions comparable to our dependent variables and we calculated power to detect GxE associations of .791 and .585 for our high and average environmental levels, respectively. These estimates support the power of the high environment category and provide fairly weak support for the power of the average environmental category. However, both estimates are well above the very low power estimates described by Duncan & Keller (2011). Further, Pluess & Belsky (2012) challenge their findings because their model assumes that there must be a main genetic association in the population; if the differential susceptibility model is applicable, then the slope should be zero in the population. As we show here, that may indeed be the case with these two loci in the dopaminergic system.
This point is exacerbated when you consider the fact that we examined 16 statistical models with 2 GxE estimates in each model. We continue to rely upon a traditional p < .05 threshold despite the multiple testing. If we used the most conservative Boferroni adjustment, then our level of significance would drop to .0015 and we would only have one interaction that met this criteria. It is important to note, however, that the likelihood of observing 11 significant interactions out of the 32 tests (assuming independence) is roughly 2.5 * 10-7. Taken together, we believe that this approach provides strong support that most or all of our GxE associations are not statistical artifacts of multiple testing. That said, we encourage readers to consider these three important limitations when considering the meaning of our findings.
Some have made very strong arguments that GxE studies should emphasize exogenous sources of environmental influence (Fletcher & Conley, 2013). If genotype and environmental exposure are correlated then it is possible that the GxE parameter estimates could be biased. We examined the possibility of gene-environment correlation by comparing the mean genotype across the three levels for each of our environmental measures. For both genes, we only observe one small departure from independence with respect to the minor allele frequency across environmental levels. In the case of DRD2 we estimated a mean genotype of .39 in the low school measure but .52 and .48 in the average and high school levels, respectively. This difference was not statistically significant (p<.053) but still worth reporting. To further investigate this point, we estimated regression models with main environmental effects that included genotype and compared those to models without genotype. If gene-environment correlation was contaminating our environmental scores then the effect of the environment would be reduced upon the inclusion of these controls. In no case did we observe this pattern. As such, we are confident that our GxE associations are not systematically biased by the subjective nature of our environmental measures.
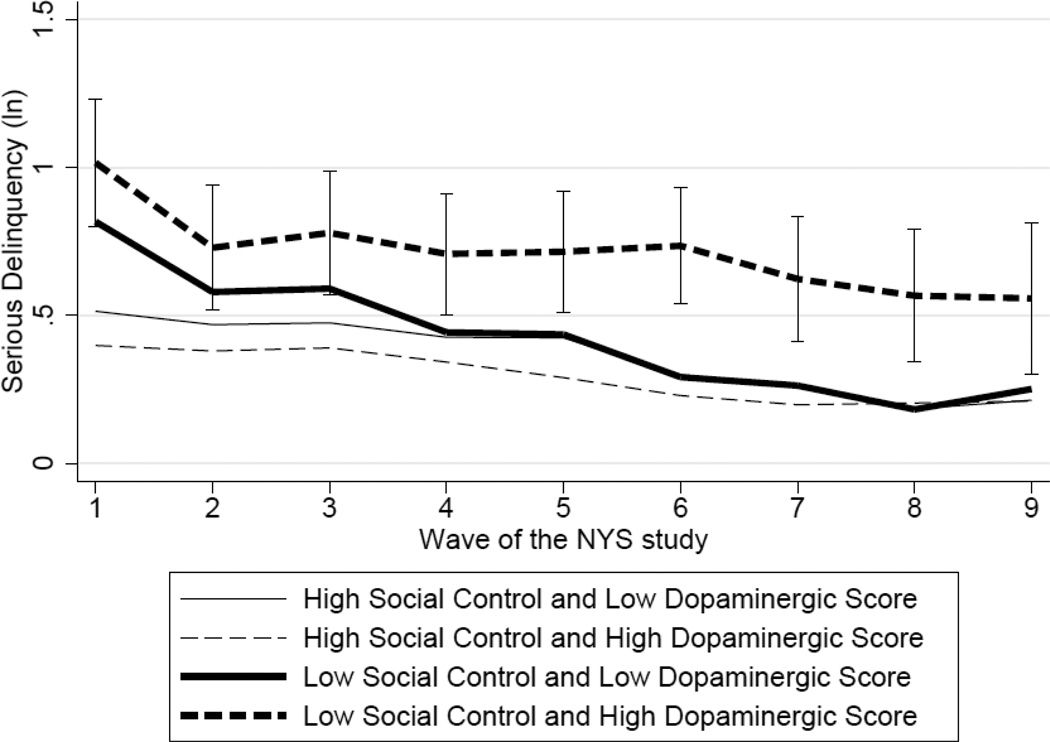
Finally, while this paper uses repeated assessments of delinquent behavior, we did not investigate the extent to which social and genetic risks combine to place individuals on separate trajectories of delinquent behavior throughout the life course. We prioritized testing the different GxE models which provided important information but it compromised our ability to test specific turning points, critical times during the life course, and the extent to which persistence or desistence are best captured by GxE associations rather than mean delinquency levels (Sakai et al., 2009). To briefly demonstrate the significance of this perspective, consider the values presented in Figure 4. Here, we estimated comparable multilevel models and we examined wave specific estimates for four groups: those with 1) protective social factors (average or low risk in the previous analyses) and low dopaminergic risk (0, 1, or 2 dopaminergic alleles); 2) protective social factors and high dopaminergic risk (3 or 4 dopaminergic alleles); 3) no protective social factors and low dopaminergic risk; and 4) no protective factors and high dopaminergic risk. Three important relationships are evident in Figure 4 and are worth describing. First, the absence of protective social factors is an important factor related to the risk of serious delinquency in adolescence regardless of genotype. Both of the darker lines are significantly higher than the corresponding lines below. Second, as we saw in models predicting differential susceptibility, those with the highest dopaminergic score in the controlled environments have the lowest levels of serious delinquency for most of the study. The third point, however, is the most obvious and perhaps the most important. While those from the least supportive environments do not differ from one another as a function of genotype in the first 3 waves of the study (as evidence by the confidence interval estimates), by Wave 4 (average age ∼17) those with lower dopaminergic scores show strong desistence patterns and then are eventually indistinguishable from those from less risky social contexts. However, those from risk environments with higher dopaminergic scores demonstrate significantly higher levels of serious delinquency that persists through young adulthood. Subtle and seemingly insignificant differences early in the life course that are due to genetic antecedents have tremendous consequences for one of two paths: a) desisting from delinquent behaviors in way that is concordant with most youth or b) maintaining these behaviors for a large part of one’s adult life. Given the timing of this departure with on time graduation from high school, we encourage future researchers to examine specific combinations of social and genetic risk factors that may place children on certain academic tracks or increase their likelihood of dropping out of school altogether.
Figure 4. Serious delinquency across the life course as a function of social controls and genetic risk.
Note: Estimates derived from a multilevel model in which observations are nested within individuals. Wave specific estimates of serious delinquency are calculated for the different combinations of social risk and genetic risk using the margins command in STATA 12.0. Confidence intervals are presented for the wave specific estimates of the high social risk and high genetic risk group (n=44) as a means of comparison to the low social risk-low genetic risk (n=436), low social risk-high genetic risk (n=138), and high social risk-low genetic risk (n=106) groups. High social risk is those who have no social controls and high genetic risk is those with 3 or 4 dopaminergic alleles. There was no bivariate association between environment and genetic risk (chi2 = 1.53, df = 1, p<.216).
Conclusion
In sum, our paper provides an opportunity to extend GxE studies linking social context, genetic factors, and delinquent behavior (Guo et al., 2008; Beaver et al., 2009; Vaughn et al., 2009; Simons et al., 2011; Vaske et al., 2011 ) using an independent sample with a long assessment period and detailed information across the typical delinquent life course. For the most part, our results are line with this previous work. That is we show evidence for both social control (Guo et al., 2008) and some support for the differential susceptibility model (Simons et al., 2011) and we show evidence for an interactive risk with the 10R allele in the DAT1 gene that was not reported in previous work. Although very similar to one another, the diathesis-stress and social control models differ from one another in terms of whether they focus on “good” (controlled) or “bad” (stressful) environments. The most consistent findings to emerge was that the absence of protective factors from schools and families rather than the presence of risk factors from friends and neighbors, appear to have the most important influence on genetic associations for delinquent behavior. As such, we find more evidence for the importance of “good” social factors for reducing the link between genotype and undesirable behavior rather than the importance of “bad” environments as a trigger for latent genetic tendencies. This contribution is somewhat subtle but it has important implications for GxE research because very little attention is paid to genetic associations across the environmental spectrum.
It is our hope that future studies examine the extent to which environments change over time to see whether the apparently weaker interactions involving, for example, neighborhood and peer group are a result of attenuated relationships—findings that may have resulted from excluding the dynamic aspect of changing environments over the life course in our analysis. Acknowledging that there is much more to be done, this paper nonetheless highlights the importance of evaluating previously published associations within the social sciences using independent samples which, to date, remain less common compared to other disciplines. Even reports of null and alternative findings are critical to the process of identifying genes and pathways by which social environments may interact with genes to create delinquent behavior. We hope that future work will build on this and other emerging studies in this important area of research.
Table 2.
Descriptive statistics for all variables used in the analysis
| Mean/% | SD/N | |
|---|---|---|
| Genotype | ||
| DAT1 (10R/10R) | 0.61 | 441 |
| (10R/9R) | 0.34 | 248 |
| (9R/9R) | 0.05 | 34 |
| DRD2 (A1/A1) | 0.06 | 43 |
| (A1/A2) | 0.34 | 246 |
| (A2/A2) | 0.60 | 435 |
| Sociodemographic and background controls | ||
| Male | 0.59 | 427 |
| Non-White | 0.16 | 116 |
| Parent college graduate | 0.35 | 253 |
| Parent unemployed | 0.06 | 43 |
| Parent below poverty | 0.16 | 116 |
| Household Size | 5.10 | 1.69 |
| GPA | 2.68 | 0.71 |
| Social control mechanisms | ||
| Family closeness | 20.04 | 2.90 |
| School attachment | 21.96 | 3.31 |
| Social risk mechanisms | ||
| Neighborhood disorganization | 7.29 | 1.85 |
| Peer delinquency | 16.65 | 5.73 |
Note: Data come from 9 waves of the National Youth Survey Family Study (NYSFS). N = 724. Cell entries represent means and standard deviations (for continuous variables) and percents and N size for categorical variables.
References
- Anchordoquy HC, McGeary C, Liu L, Krauter KS, Smolen A. Genotyping of three candidate genes following whole genome preamplification of DNA collected from buccal cells. Behavior Genetics. 2003;33(1):73–78. doi: 10.1023/a:1021007701808. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Wright JP, Boutwell BB, Barnes JC, DeLisi M, Vaughn MG. Exploring the association between the 2-repeat allele of the MAOA gene promoter polymorphism and psychopathic personality traits, arrests, incarceration, and lifetime antisocial behavior. Personality and Individual Differences. 2013;54:164–168. [Google Scholar]
- Beaver KM, DeLisi M, Wright JP, Vaughn MG. Gene-environment interplay and delinquent involvement: Evidence of direct, indirect and interactive effects. Journal of Adolescence Research. 2009;24:147–168. [Google Scholar]
- Beaver KM, Wright JP, DeLisi M, Daigle LE, Swatt Marc L, Gibson CL. Evidence of a gene x environment interaction in the creation of victimization. International Journal of Offender Therapy and Comparative Criminology. 2007;51:620–645. doi: 10.1177/0306624X07304157. [DOI] [PubMed] [Google Scholar]
- Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. The Journal of Child Psychology and Psychiatry. 2011;52:619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Boardman JD. State-level moderation of genetic tendencies to smoke. American Journal of Public Health. 2009;99(3):480–486. doi: 10.2105/AJPH.2008.134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Blalock CL, Pampel FC. Trends in the genetic influences on smoking. Journal of Health & Social Behavior. 2010;51(1):108–123. doi: 10.1177/0022146509361195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Barnes LL, Wilson RS, Evans DA, Mendes de Leon CF. Social disorder, APOE-E4 genotype, and change cognitive function among older adults living in Chicago. Social Science & Medicine. 2012;74:1584–1590. doi: 10.1016/j.socscimed.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Roettger ME, Domingue BW, McQueen MB, Haberstick BC, Harris KM. Gene-environment interactions related to body mass: school policies and social context as environmental moderators. Journal of Theoretical Politics. 2012b;24(3):370–388. doi: 10.1177/0951629812437751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brame R, Paternoster R. Missing Data Problems in Criminological Research: Two Case Studies. Journal of Quantitative Criminology. 2003;19(1):55–78. [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harington H, McClay J, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WP, Van der Does AJ, Hommel B. Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience. 2010;170:782–788. doi: 10.1016/j.neuroscience.2010.07.050. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, Grant C, et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10:686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot DS, Huizinga D, Menard S. Multiple Problem Youth: Delinquency, Drugs and Mental Health. New York: Springer-Verlag; 1989. [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary-neurodevelopment theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Farrington DP, Gundry G, West DJ. The familial transmission of criminality. Medicine, Science, and the Law. 1975;15:177–186. doi: 10.1177/002580247501500306. [DOI] [PubMed] [Google Scholar]
- Ferguson CJ. Genetic contributions to antisocial personality and behavior: A meta-analytic review from an evolutionary perspective. The Journal of Social Psychology. 2010;150:160–180. doi: 10.1080/00224540903366503. [DOI] [PubMed] [Google Scholar]
- Freese J Shostak S. Genetics and Social Inquiry. Annual Review of Sociology. 2009;35:107–128. [Google Scholar]
- Frisell T, Lichtenstein P, Långström N. Violent crime runs in families: a total population study of 12.5 million individuals. Psychological Medicine. 2011;41:97–105. doi: 10.1017/S0033291710000462. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- Gottfredson MR, Hirschi T. A General theory of crime. Stanford, CA: Stanford University Press; 1990. [Google Scholar]
- Gouldner AW. The norm of reciprocity: a preliminary statement. American Sociological Review. 1960;25(2):161–178. [Google Scholar]
- Groman SM, Jentsch JD. Cognitive control and the dopamine D2-like receptor: a dimensional understanding of addiction. Depression and Anxiety. 2011;29(4):295–306. doi: 10.1002/da.20897. [DOI] [PubMed] [Google Scholar]
- Guo G, Cai T, Guo R, Wang H, Harris KM. The Dopamine Transporter Gene, a Spectrum of Most Common Risky Behaviors, and the Legal Status of the Behaviors. PLoS ONE. 2010;5(2):e9352–000. doi: 10.1371/journal.pone.0009352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Roettger ME, Shih JC. Contributions of the DAT1 and DRD2 genes to serious and violent delinquency among adolescents and young adults. Human Genetics. 2007;126:125–136. doi: 10.1007/s00439-006-0244-8. [DOI] [PubMed] [Google Scholar]
- Guo G, Roettger ME, Cai T. The integration of genetic propensities into social-control models of delinquency and violence among male youths. American Sociological Review. 2008;73:569–588. [Google Scholar]
- Haberstick BC, Smolen A. Genotyping of three single nucleotide polymorphisms following whole genome preamplification of DNA collected from buccal cells. Behavioral Genetics. 2004;34(5):541–7. doi: 10.1023/B:BEGE.0000038492.50446.25. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- Huizinga D, Haberstick BC, Smolen A, Menard S, Young SE, Corley RP, Stallings MC, et al. Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotype. Biological Psychiatry. 2006;60(7):677–83. doi: 10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, early adversity, and gene-environment interaction predicting children’s mental health: New evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. Journal of Health & Social Behavior. 1995;35:80–94. [PubMed] [Google Scholar]
- Marino C, Vanzin L, Giorda R, Frigerio A, Lorusso ML, Nobile M, Molteni M. Measures of childhood problem behaviors and DRD2/Taq1 and DRD4/48bp-repeat polymorphisms. Behavior Genetics. 2004;34:495–502. doi: 10.1023/B:BEGE.0000038487.80597.7e. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology: Gene - environment interplay in antisocial behavior. Psychological Bulletin. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Craig IW, McGuffin P. Behavioral Genetics in the Postgenomic Era. Washington, D.C: American Psychological Association; 2008. [Google Scholar]
- Pluess M, Belsky J. Conceptual Issues in Psychiatric Gene-Environment Interaction Research: A comment. Am J Psychiatry. 2012;169:222–223. doi: 10.1176/appi.ajp.2011.11111614. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Reiss D Leve L.D. Genetic expression outside the skin: Clues to mechanisms of Genotype x Environment interaction. Development and Psychopathology. 2007;19:1005–1027. doi: 10.1017/S0954579407000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, et al. Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression: A Meta-analysis. JAMA. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins L. Deviant children grown up. Baltimore, MD: Williams and Wilkens; 1966. [Google Scholar]
- Robison SM. Can Delinquency Be Measured? New York: Columbia University Press; 1936. [Google Scholar]
- Rutter M. Genes and behavior: Nature-nurture interplay explained. Malden, MA: Blackwell; 2006. [Google Scholar]
- Sakai JT, Boardman JD, Gelhorn H, Smolen A, Corley RP, Menard S, Huizinga D, et al. Utilizing trajectory analyses to refine phenotype for genetic associations: conduct problems and 5HTTLPR. Psychiatric Genetics. 2010;20(5):199–206. doi: 10.1097/YPG.0b013e32833a20f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson RJ, Laub JH. Crime in the Making: Pathways and Turning Points Through Life. Cambridge: MA: Harvard University Press; 1993. [Google Scholar]
- Shanahan MJ, Hofer SM. Social Context in Gene-Environment Interactions: Retrospect and Prospect. Journals of Gerontology: Series B. 2005;60B:65–76. doi: 10.1093/geronb/60.special_issue_1.65. Special Issue. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Boardman JD. Genetics and behavior in the life course: a promising frontier. In: Elder Glen H, Jr, Giele Janet Z., editors. The Craft of Life Course Research. London: Guilford; 2009. pp. 215–235. [Google Scholar]
- Simons RL, Lei MK, Beach SRH, Brody GH, Philibert RA, Gibbons FX. Social Environment, Genes, and Aggression: Evidence Supporting the Differential Susceptibility Perspective. American Sociological Review. 2011;76:883–912. doi: 10.1177/0003122411427580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Thornberry TP. Toward an interactional theory of delinquency. Criminology. 1987;25(4):863–892. [Google Scholar]
- Turkheimer E. Three laws of behavior genetics and what they mean. Current Directions in Psychological Science. 2000;9:160–164. [Google Scholar]
- Vaske J, Wright JP, Beaver KM. A Dopamine Gene (DRD2) Distinguishes between Offenders Who Have and Have Not Been Violently Victimized. International Journal of Offender Therapy and Comparative Criminology. 2011;55:251–267. doi: 10.1177/0306624X10361583. [DOI] [PubMed] [Google Scholar]
- Vaughn MG, DeLisi M, Beaver KM, Wright JP. DAT1 and 5HTT Are Associated With Pathological Criminal Behavior in a Nationally Representative Sample of Youth. Criminal Justice and Behavior. 2009;36:1113–1124. [Google Scholar]
- Wadsworth MEJ. Delinquency. Pulse rate and early emotional deprivation. British Journal of’ Criminology. 1976;6:245–250. [Google Scholar]
- Wilson FD, Smoke GL, Martin JD. The replication problem in sociology: a report and a suggestion. Sociological Inquiry. 1973;43(2):141–149. [Google Scholar]
- Zhang L, Cui X, Schmitt K, Hubert R, Navidi W, Arnheim N. Whole-genome amplification from a single cell: implications for genetic analysis. Proceedings of the National Academy of Sciences. 1992;89:5847–5851. doi: 10.1073/pnas.89.13.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]