Abstract
Neurotrophins are a family of growth factors that have been found to be central for the development and functional maintenance of the nervous system, participating in neurogenesis, neuronal survival, axonal growth, synaptogenesis and activity-dependent forms of synaptic plasticity. Trauma in the adult nervous system can disrupt the functional circuitry of neurons and result in severe functional deficits. The limitation of intrinsic growth capacity of adult nervous system and the presence of an inhospitable environment are the major hurdles for axonal regeneration of lesioned adult neurons. Neurotrophic factors have been shown to be excellent candidates in mediating neuronal repair and establishing functional circuitry via activating several growth signaling mechanisms including neuron-intrinsic regenerative programs. Here, we will review the effects of various neurotrophins in mediating recovery after injury to the adult spinal cord.
Keywords: Axonal guidance, neurotrophin, regeneration, functional recovery, sprouting
Introduction
Trauma to the adult mammalian central nervous system (CNS) often leads to devastating clinical consequences without the prospects of complete functional recovery, usually due to the failure of spontaneous nerve regeneration. This failure could be attributed to both the absence of intrinsic regenerative potential and the inhospitable environment, including the lack of growth-supporting molecules and the presence of growth-inhibitory factors after traumatic injury. Neurotrophins (NTs) have emerged as promising extrinsic factors to augment nerve regeneration after injury. Substantial progress has been made in identifying the roles of NTs, in promoting nerve regeneration and functional recovery after injury.
NTs are a family of growth factors found to be central for the development and functional maintenance of the nervous system, participating in neurogenesis, neuronal survival, axonal growth, synaptogenesis and activity-dependent forms of synaptic plasticity (Lu et al., 2005). Four members of the NT family have been identified in mammals: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3) and neurotrophin 4/5 (NT4/5) (Chao, 2003a). All four neurotrophins are initially translated as proneurotrophins and are cleaved subsequently by proteolysis to produce 13 kDa proteins that exist as noncovalently linked homodimers (Bibel and Barde, 2000).
The effects of NTs are mediated through signaling by two distinct classes of neurotrophin receptors (NTRs): the p75 pan-neurotrophin receptor (p75NTR) and tropomyosin related kinase (Trk) receptor tyrosine kinases. The latter consists of three members: TrkA, TrkB, and TrkC, which preferentially binds to NGF, BDNF & NT4/5, and NT3, respectively (Chao, 2003a). Ligand binding to Trk receptors triggers receptor dimerization and autophosphorylation of tyrosine residues in the intracellular domain, followed by the activation of diverse signaling cascades, such as the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), Ras/mitogen-activated protein kinase (MAPK), and phospholipase C-γ (PLC-γ) pathways. These pathways play important roles in neuronal survival, differentiation, and synaptic plasticity (Blum and Konnerth, 2005) (Fig. 1).
Figure 1.
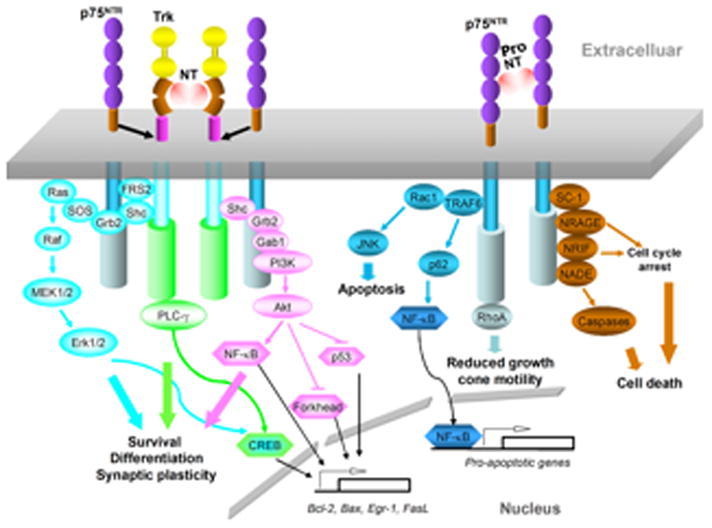
Signaling cascades mediated by Trk receptors and p75NTR. Trk family members recruit and increase the phosphorylation of PLC-γ and Src homologous and collagen-like adaptor protein (Shc), which leads to activation of PI3K and Erk, and participate in cell survival, differentiation, and synaptic plasticity, respectively. Collaboration of p75NTR with Trk augments the Trk effects. In the absence of Trk, p75NTR predominantly signals to activate NF-κB and Jun N-terminal kinase (JNK), and modulates RhoA activity. Pro-neurotrophins also preferentially bind to the p75NTR signaling apoptotic pathways. These responses are mediated through adaptor proteins that bind to the cytoplasmic domain of p75, including neurotrophin-receptor interacting factor (NRIF), neurotrophin-associated cell death executor (NADE), neurotrophin-receptor-interacting MAGE homolog (NRAGE), and Schwann cell 1 (SC1), which can exert effects on apoptosis, growth cone collapse and cell cycle arrest. Akt, protein kinase B; FRS2, fibroblast growth factor receptor substrate 2; Gab1, Grb2-associated binder-1; Grb2, growth factor receptor-bound protein 2; MEK, mitogen-activated protein kinase (MAPK)/Erk kinase; SOS, Son of Sevenless; TRAF6, tumor necrosis factor receptor-associated factor 6.
p75NTR is the co-receptor that refines Trk affinity and specificity for NTs (Huang and Reichardt, 2003; Kuruvilla et al., 2004) and augments Trk signaling (Epa et al., 2004). In the absence of Trks, p75NTR signals activate nuclear factor-κB (NF-κB), Jun N-terminal kinase (JNK), and modulate RhoA activity, which are related to cell survival, death and neurite outgrowth, respectively (Chao, 2003b) (Fig. 1).
NTs are widely expressed in both the peripheral and central nervous system during development and adulthood (Lessmann et al., 2003). The expression of neurotrophins in the adult is comparatively low because of reduced requirement for trophic support and axonal plasticity. The expression of NTs are increased after injury (Tonra et al., 1998), indicating the possible role of NTs in nerve regeneration after traumatic CNS injury. Regeneration of peripheral nervous system after an injury is attributed to the dramatic upregulation in the expression of NT s by Schwann cells (Höke et al., 2006). These dedifferentiated Schwann cells act as one of the best cellular substrates to promote axonal regeneration (Lehmann and Höke, 2010).
NT treatment induce sensory axonal regeneration after dorsal root lesions
After injury of their central processes, dorsal root ganglion (DRG) neurons exhibit a robust and generally successful regenerative response within the peripheral dorsal root; however, at the PNS/CNS transition zone, known as the dorsal root entry zone (DREZ), axonal regeneration fails resulting in sensory loss. Regenerative failure at the DREZ is thought to be caused by the presence of growth-inhibitory cues (Kim et al., 2004; Domeniconi and Filbin, 2005), the absence of growth-supporting factors (Ide, 1996), or the lack of intrinsic regenerative capacity of cut axons. Neurotrophin treatments are sufficient for functional regeneration of selective sensory axons after dorsal root injury (Romero et al., 2001).
Initial regeneration of sensory axons into the adult spinal cord with NT treatment showed limited success as a therapeutic strategy (Ramer et al., 2000). In their experiments, rats received complete crush injuries on two or three cervical dorsal roots, and osmotic mini-pumps were instantly implanted to infuse neurotrophic factors intrathecally during the next 7 days. Some of the damaged axons selectively regenerated across the DREZ with the trophic support of NGF, GDNF and NT3. In rats treated with NGF and NT3, dorsal horn neurons were found to establish functional synaptic connectivity with regenerating axons when tested by peripheral nerve stimulation. Behavioral assessments showed some functional recovery corresponding to the modalities of sensory neuron subpopulations (Ramer et al., 2000). Thus, NT treatment may serve as a viable treatment in promoting nerve regeneration and functional recovery from root avulsion injuries.
Application of neurotrophins using osmotic minipumps is limited to the intrathecal space and does not support gradient production of NTs by cells within the spinal cord itself. Recombinant viral-vector based gene expression represents a more effective method to induce long-term, robust expression of NTs within an endogenous cell population in a spatially and temporally restricted manner (Smith and Romero, 1999). To induce axonal regeneration into the spinal cord, recombinant adenoviruses were microinjected within the spinal cord along the DREZ to express NGF or other growth factors by endogenous glia 16 days after injury. This NT treatment induced robust axonal regeneration across the DREZ and led to near-normal recovery of thermal-nociceptive sensory function, suggesting that NT gene therapy may serve as a useful treatment to elicit recovery after dorsal root avulsion (Romero et al., 2001). This treatment, however, also resulted in extensive sprouting of non-injured sensory axons, which could cause hyperalgesia and chronic pain (Romero et al., 2001). Neurotrophin induced sprouting of non-injured axons can have both positive and negative effects, in which sprouting that enhances and refines potential motor circuit relays could enhance functional recovery (Bonner et al., 2010), whereas, sprouting which effects pain or autonomic pathways could be detrimental (Romero et al., 2001; Cameron et al., 2006). To reduce hyperinnervation and sprouting of pain pathways and to confine axonal regeneration to “normal” territories, gradients co-expressing NGF dorsally and the chemorepulsive factor Semaphorin-3A ventrally were established. In this overlapping gradient, NGF provided growth-support for axons regenerating through the DREZ, but as they grew ventrally within the spinal cord, they encountered an ever increasing concentration of Semaphorin-3A which prevented growth into ventral territories (Tang et al., 2004b). This study shows that combinations of growth supportive and growth-inhibitory molecules can be strategically supplied to promote regeneration, while controlling targeting of axons.
NT treatment increase regeneration after spinal cord injury (SCI)
Trauma to the spinal cord usually severs axons, disrupts microvasculature, kills neurons and glia, and induces the formation of a dense glial-fibroblastic scar, resulting in a permanent impairment or loss of sensory and motor functions. The damaged axons proximal to the lesion form retraction bulbs and display considerable die-back away from the lesion site (Coumans et al., 2001). Severed neurons show ongoing and progressive apoptosis and the surviving ones make such a limited spontaneous attempt to regrow that the regenerating axons fail to enter or traverse the lesion area (Cajal, 1928). Some functional recovery can be achieved by NT treatments. In the injured spinal cord, neurotrophin delivery by various methods supports the growth of discrete neuronal populations. For example, NGF supports the sprouting and regeneration of cholinergic local motor axons, primary nociceptive axons, and cerulospinal axons (Tuszynski et al., 1996; Grill et al., 1997; Romero et al., 2001; Tang et al., 2004a). Similarly, BDNF secreting bone marrow stromal cell transplants enhance regeneration of multiple neuronal populations including raphaespinal, cerulospinal, rubrospinal, local motor and propriospinal axons, while NT3 expression promotes regeneration of ascending sensory neurons across the DREZ and within the dorsal columns (Hollis and Tuszynski, 2011). Several studies show that the location of neurotrophin expression can also determine the extent of regeneration (Lu et al., 2001; Taylor et al., 2006). This was demonstrated using a combination of sciatic nerve conditioning lesion, bone marrow stromal cells (MSCs) and lentiviral NT-3 gene transfer distal to a spinal cord lesion site, allowing the ascending dorsal column sensory axons to regenerate across the lesion site (Taylor et al., 2006). In contrast, delivery of NT secreting MSC within the lesion site promoted regrowth into but not beyond the lesion site (Lu et al., 2003). More recent studies have indicated that the above combination also promoted regeneration of dorsal column sensory axons after chronic injury (Kadoya et al., 2009).
Although the above approaches promote sensory axon regeneration within the adult spinal cord, neurotrophin mediated regeneration of supraspinal axons appears dramatically lower. Eight weeks after cervical SCI, neurons within the red nucleus (RSNs) undergo significant atrophy, but do not completely degenerate. Numerous studies show treatment with BDNF and/or NT3 immediately after injury significantly enhanced survival or reduced atrophy. Delayed treatment for eight weeks with a combination of BDNF and NT3 resulted in almost complete recovery of the neurons within the red nucleus (Novikova et al., 2000). Likewise, BDNF delivery to this brainstem nucleus even 1 year after SCI fully reversed the atrophy of motor neurons in the red nucleus (Kwon et al., 2002; Kwon et al., 2007). In general the application of NTs alone to the injury site rarely results in regeneration of rubrospinal axons; however many studies have shown regeneration when combined with other treatments. Several studies have shown that co-application of either BDNF or NT-3 with growth supportive transplants of either Schwann cells, peripheral nerve, or fetal transplants induce some regeneration of rubrospinal axons into the grafts, but not out of grafts and back into host tissue (Xu et al., 1995; Kobayashi et al., 1997; Ye and Houle, 1997; Iarikov et al., 2007). Similar results have also been observed with transplants of fibroblasts transduced with these NTs (Tobias et al., 2003). Interestingly, the application of BDNF and NT-3 distal to the transplant promoted outgrowth of axons from Schwann cell grafts back into the host spinal cord (Bamber et al., 2001).
In humans, the rubrospinal pathway is considered rudimentary, whereas, the corticospinal pathway is considered the primary voluntary motor pathway. After injury to the corticospinal tract (CST), lesioned cortical motor neurons begin to atrophy (Brock et al., 2010; Nielson et al., 2011). Preventing cortical neuronal atrophy and activating intrinsic regenerative mechanisms might be necessary for long distance CST regeneration. Infusion of BDNF into the motor cortex can apparently activate regenerative responses as evident by enhanced collateral sprouting in the injured spinal cord (Vavrek et al., 2006). BDNF also reversed cortical motor neuron atrophy in rodents and primates when provided at the spinal cord lesion site (Brock et al., 2010). In contrast, transplantation of fibroblasts expressing BDNF or NT 4/5 in the spinal cord failed to increase sprouting after a midthoracic injury (Lu et al., 2001; Blesch et al., 2004). In a recent study, lentiviral vectors were used to overexpress TrkB receptors in corticospinal motor neurons and after subcortical axotomy, corticospinal axons regenerated into the lesion site expressing BDNF (Hollis et al., 2009). In general, the CST is the most difficult tract to induce to regenerate and many more studies are needed to better characterize the regenerative prospects for the spectrum of neurotrophic factors available.
After CNS injury, inhibitory extracellular matrix (ECM) molecules, such as chondroitin sulfate proteoglycans (CSPGs) are upregulated and inhibit neurite outgrowth. Transplantation of NGF gene-modified fibroblasts into a spinal cord lesion promoted robust axonal regeneration into grafts despite the dense labeling of the CSPG NG2 (Jones et al., 2003). Notably, cellular sources of NG2 within the lesion site also produced the cell adhesion molecule L1 and laminin. Thus, with NGF treatment, axons grow along substrates co-expressing both inhibitory and permissive molecules, indicating the participation of NGF in mediating the balance between local permissive and inhibitory signals for successful axonal regeneration (Jones et al., 2003). Degradation of glycosaminoglycan side chain of CSPG by local administration of chondroitinase ABC (ChABC) can enhance local sprouting and facilitate axonal regeneration by reducing the effect of the inhibitory milieu (Galtrey et al., 2008; Bartus et al., 2012). When ChABC-trehalose (a thermostabilized form of ChABC) was co-administered with NT-3 in an agarose gel scaffold within the lesion site, increased sprouting of serotonergic axons and cholera toxin B positive axons were observed (Lee et al., 2010). Similarly, transplanted adult DRG neurons near CSPG rich dorsal column nuclei showed a 10-fold increase in growth potential with simultaneous application of both ChABC and NT-3, when compared to application of ChABC or NT-3 alone, indicating a co-operative interaction between neurotrophins and disinhibitory mechanisms (Massey et al., 2008). In summary, removal of extracellular inhibitory components in conjunction with availability of NTs appears to have a synergistic effect on regeneration in several injury models.
Neurotrophins and myelination
Many of the physiologic and behavioral deficits after contusive SCI are attributed to demyelination of axons. A vital strategy for enhancing recovery from contusive SCI is remyelination. In vitro, NGF and BDNF exhibit a promyelinating influence on DRG explants via activation of neuronally expressed TrkA and oligodendroglial TrkB-FL receptors (Chan et al., 2004; Xiao et al., 2010) respectively. In contrast, in vitro, exogenous NT-3 has been shown to have an inhibitory effect on Schwann cell dependent myelination (Chan et al., 2001; Cosgaya et al., 2002). Latter studies found a promyelinating influence of NT3 via a proliferative response on oligodendrocyte progenitor cells (OPCs) (Xiao et al., 2009). Similarly, heterozygous knock outs of NT3 displayed a reduction in peripheral myelin mRNA and protein levels suggesting a promyelinating role for NT3 (Woolley et al., 2008). Myelination was also enhanced by transplantation of multi-neurotrophin-expressing glial-restricted precursor cells (GRPs) (Cao et al., 2005). In vitro experiments showed that NT3 and BDNF-treatments enhanced proliferation and survival of embryonic spinal cord-derived GRPs, which differentiated into both oligodendrocytes and astrocytes, and formed central myelin around axons of cultured neurons. Gene-modified GRPs expressing both BDNF and NT3 were transplanted into the contused adult thoracic spinal cord, and differentiated into mature oligodendrocytes and formed morphologically normal-appearing myelin sheaths around the axons, resulting in recovery of some motor function (Cao et al., 2005). Therefore, combined treatment with NTs and GRP within grafts may prove to be a useful therapeutic strategy to repair spinal cord injury caused by axonal demyelination. Likewise, adipose derived stem cells (ASCs) produce NGF and BDNF along with several other growth factors, and the myelination master gene Krox-20 (Erg2) together with components encoding peripheral myelin. Transplantation of ASCs resulted in enhanced functional recovery of crushed motor and sensory fibers, most likely due to their ability to produce NTs and myelin precursor proteins, which act complementary to promote recovery (Lopatina et al., 2011). Another study reported that the transplantation of neural stem cells (NSCs) expressing NT3/D15A (a multi NT with the capacity to bind both TrkC and TrkB) enhanced partial hindlimb recovery and myelination in the chronic phase of spinal cord injury (Kusano et al., 2010). These studies suggest that functional recovery by NTs could be mediated by a dual mechanism supporting both axonal regeneration and remyelination.
NT treatments enhance neuronal survival and repair after traumatic brain injury (TBI)
Traumatic brain injury (TBI) is a complex process generalized into four categories including primary injury, secondary injury, inflammatory response and repair-regeneration (Ray et al., 2002). Ongoing and progressive cell death and diffuse axonal injury are hallmark features of TBI. Restorative and regenerative strategies that have focused on enhancing the survival of injured neurons and replacing dysfunctional and dead cells with NT treatments have received broad attention in the therapy of TBI.
In fluid-percussion TBI rats, continuous infusion of NGF to the cerebral ventricle enhanced activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) whereas the level of calcium ([Ca2+]i) decreased when compared to the trauma only group (Zhou et al., 2003). This suggested that exogenous NGF attenuated the injury to neurons induced by oxygen free radicals and reduced the severe overload of [Ca2+]i. Liposome-mediated NGF cDNA intraventricular transfection following TBI attenuated the loss of cholinergic neuronal immunostaining in the rat septum (Zou et al., 1999), showing the neuroprotective effect of NGF after TBI. After lateral fluid-percussion brain injury, fetal cortical grafts were stereotactically transplanted into the injury cavity in the absence or presence of continuous NGF infusion. Although all transplantation group showed the same level of grafted tissue survival and improved neurological motor function, only the groups with NGF infusion demonstrated significant improvement in memory scores (Sinson et al., 1996). Despite the promising results that have been obtained from transplanting fetal tissue in TBI therapy, technical and ethical concerns over the use of fetal tissue as source for transplantation have been raised and alternative sources for cellular transplantation are currently under investigation. The NT2 cell line, a human embryonic teratocarcinoma line, can be pretreated in vitro to differentiate into NT2N neurons (Trojanowski et al., 1997) a transplant source. Longhi and colleagues (2004b) transplanted NT2N neurons, with or without ex vivo NGF gene therapy, into the medial septum of adult mice following controlled cortical impact (CCI) brain injury. At 1 month post-transplantation, animals engrafted with NGF gene modified NT2N neurons showed a significantly improved learning ability with no significant difference in motor function compared to brain-injured mice receiving untransduced NT2N neurons (Longhi et al., 2004a). As with fetal transplants, this data suggests combined neuronal replacement and neurotrophin therapy may selectively improve cognitive function following TBI. Clinically, higher NGF has been shown to be associated with better neurologic outcome in phase 2 of TBI in pediatric patients (Chiaretti et al., 2008).
Cellular grafts employed for regenerative therapy should meet at least four requirements, including long-term survival, migrating to appropriate destination, differentiating into appropriate cell types and reconnecting with the host tissues. Another candidate cell, HiB5 cells are conditionally immortalized neural progenitor cells derived from an embryonic rat hippocampus with the differentiation potential for both neuron and glia (Kim et al., 2002). HiB5 cells with or without NGF gene-modification were transplanted stereotactically into several individual sites adjacent to the injury site after TBI, eliciting significant improvements in neuromotor function. However, only the animals receiving NGF gene-modified HiB5 cells showed significant reduction in hippocampal CA3 cell death (Philips et al., 2001). Transplanted DRG neurons can cross a lesion in the corpus callosum along a gradient pathway of NGF extending through the lesion (Jin et al., 2008). Similarly, transplantation of bone marrow stromal cells overexpressing BDNF attenuated neuronal injury in TBI models as indicated by improvement in behavioral recovery (Wang et al., 2013).
Bone marrow stromal cells (MSCs) constitute a heterogeneous cell populations in the bone marrow, supporting the growth and differentiation of hematopoietic stem cells. Previously, it has been reported that MSCs have stem-cell-like characteristics that allow them to differentiate into different cell types, including neurons and glia (Chu et al., 2004). The convenience of isolating MSC from injured animals or patients makes it a potential clinical tool for cellular replacement. Pretreatment of MSCs with BDNF or NGF before intracerebral transplantation into adult rats after TBI significantly increased the number of engrafted cells accompanied by significantly improved motor function (Mahmood et al., 2002), suggesting the therapeutic potential of combining MSC implantation and NT treatments.
Strategies for NT treatments
Different strategies for NT treatments, such as NT protein infusion or NT gene therapy, might show different effects for nerve injury repair. Carefully considering the advantages and disadvantages of NT treatment strategies and choosing an appropriate one for neurotrauma therapy is necessary. Below are some common strategies for NT treatments.
Implantation of NT-soaked gelfoam
Gelfoam is absorbable gelatin sponge, which is usually used as a hemostatic device. As gelfoam is capable of absorbing and holding solution within its meshwork, implantation of NT-soaked gelfoam would release NT proteins slowly for a few days. Elastomeric gelatinous tube and multifilament gelatinous fibers impregnated with laminin, fibronectin, and NGF showed strong axonal regeneration potential (Gámez et al., 2003). Implantation of NT-soaked gelfoam has been proven effective for SCI and TBI repair (Royo et al., 2003; Deumens et al., 2005). However, it is difficult to use gelfoam for continuous NT treatments longer than a few days.
NT delivery by osmotic mini-pumps
Osmotic mini-pumps are often used as an alternative to repetitive injections for prolonged drug delivery in adult rats. NT infusion by osmotic mini-pumps has been commonly used in neurotrauma repair (Deumens et al., 2005). Osmotic mini-pump delivery is a good method for administration of NT proteins in a restricted spatial and temporal manner with little immune-response. Sometimes this system is combined with cellular transplantation for nerve regeneration therapy (Ferguson et al., 2001). However, such a system is inconvenient for long-term continuous NT treatments and cause much more parenchymal damage when compared to gene therapy (Kwon et al., 2007).
Injection of recombinant viruses with exogenous NT genes
With virus-mediated gene therapy, exogenous NTs could be expressed effectively in host cells for long-term treatments (Romero and Smith, 1998; Smith and Onifer, 2011). Excitingly, with specific promoters and reporters for gene expression, exogenous NTs could be expressed in specific cell types and conveniently detected. Viral injection can also be performed to generate both short and long distance gradients to target or induce long distance directed growth from subpopulation of neurons (Smith and Onifer, 2011). The major disadvantage for broad usage of virus-mediated NT gene therapy might be the immune response caused by some viruses and the inability to shut off gene expression after regeneration is complete or if secondary problems occur. However, as viral gene transfer techniques develop, better methods for transgene regulation and viral tropism will offer tighter control of gene expression within subpopulations of cells.
Implantation of gene-modified cells
Cellular transplantation acts as replacement for cell loss after injury. Neural transplantation was initially developed as a potential therapy for neurodegenerative diseases such as Parkinson disease and Huntington disease and has now begun to be evaluated in models of CNS injury (Ferraro et al., 2004). A number of tissues and cells including fetal cells, neural stem cells, Schwann cells, MSCs and olfactory-ensheathing cells have been tested in animal models of CNS injury. These cells fill cystic cavities which form after SCI and provide growth supportive substrates to bridge the lesion. Combined with NT treatments, usually with NT gene modification, cellular transplantation has proven to be an effective therapeutic strategy for neurotraumatic repair (Hendriks et al., 2004). The variability of injured cell types to be replaced is one of the major challenges of replacement therapy.
Challenges for NT treaments after injury
Inhibitory effects of NT treatments on nerve regeneration
Despite the numerous reports of NT supporting nerve regeneration after injury, inhibitory effects of NT treatments on injured nerve repair have also been reported. This issue is of important consideration when combinatorial approaches are used in treatment. For example, addition of BDNF to olfactory ensheathing cell transplants increased the lesion cavitation and worsened forelimb reaching and coordinated walking behaviors (Bretzner et al., 2008). After peripheral nerve injury, axotomized sensory neurons in the DRG upregulate BDNF expression and undergo apoptosis. Treatment with BDNF neutralizing antibodies significantly reduced apoptosis of sensory neurons in DRG explants in vitro whereas exogenous BDNF increased the percentage of apoptotic neurons within the DRG. However, treatment with antiserum to BDNF in vivo after axotomy of sciatic nerve significantly exaggerated the DRG neuronal loss (Zhou et al., 2005). Such a difference might be attributed to the different expression level of p75NTR and/or pro-neurotrophins. While endogenous BDNF induced apoptosis of DRG neurons in vitro where more neurons expressed p75NTR, it prevented apotosis in vivo where fewer neurons expressed p75NTR after sciatic nerve axotomy (Zhou et al., 2005). Moreover, BDNF acts oppositely on peripheral nerve regeneration in a dose-dependent manner. Administration of BDNF (0.5–2 μg/day for 28 days) on axotomized tibial nerve stump promoted axonal regeneration of motor neurons whose regenerative capacity had been reduced by chronic axotomy. In contrast, high doses of BDNF (12–20 μg/day for 28 days) significantly inhibited motor axon regeneration. The inhibitory action of high dose BDNF could be reversed by functional blockade of p75NTR (Boyd and Gordon, 2002). Hence, dosing of NTs could greatly effect nerve regeneration, in some cases and present a challenge for NT treatments after nerve injury. On the other hand, pro-neurotrophins have gained recognition for contributing to apoptosis (Freidman 2010). Pro-neurotrophins are uncleaved neurotrophin precursors which can be secreted by neurons and glia. Unlike fully mature forms of neurotrophins which preferentially bind Trk receptors, pro-neurotrophins preferentially bind p75NTR contributing to apoptosis (Freidman, 2010). In either situation neutralizing p75NTR binding might be a possible strategy to overcome such a challenge.
Conclusion
NTs play important roles in many facets of nerve regeneration after traumatic CNS injury. NT treatments enhanced neuronal survival, axonal regrowth, remyelination and synaptic plasticity, etc (Fig. 2). It has been widely reported that successful NT-mediated nerve regeneration after neurotrauma leads to functional recovery, either through NT protein delivery or gene therapy. Despite the challenges for NT treatments on nerve regeneration, such as reciprocal collaboration and the positive/negative action of its low-affinity receptor p75NTR, a better understanding of the molecular mechanisms and therapeutic application for NT treatments in future would absolutely broaden its clinical merit of nerve regeneration after neurotrauma.
Figure 2.
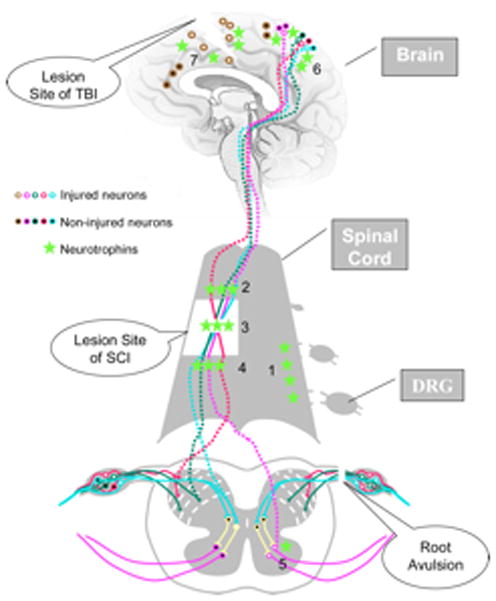
Neurotrophin treatments on nerve regeneration after neurotrauma. Traumatic injury in CNS, such as root avulsion, spinal cord injury (SCI), and traumatic brain injury (TBI), results in death of injured neurons and failure of severed axonal regeneration. Neurotrophin treatments are reported to induce sensory axons to regenerate through the DREZ after root avulsion (1) and severed axons to re-grow (2) across the lesion site (3) into distal host tissues (4), enhance neuronal survival after SCI (5) and TBI (6, 7).
Acknowledgments
This work was funded by a grant from the National Institute of Neurological Disorders and Stroke R01 NS060784 and the Shriners Hospital for Pediatric Research grants SHC 84050 and SHC 85200 (GMS).
Footnotes
Neither Dr. G.M. Smith or L. Kelamangalath have any conflicts of interest.
Compliance with ethic guidelines
All animal experiments followed guidelines and regulations for the care and use of laboratory animals and were approved by the Institutional Animal Care and Research Advisory Committee at Temple University, School of Medicine. The University Laboratory Animal Resource Facility is a AAALAC and USDA approved facility that strictly abides by NIH animal care guidelines.
References
- Bamber NI, Li HY, Lu XB, Oudega M, Aebischer P, Xu XM. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur J Neurosci. 2001;13(2):257–268. [PubMed] [Google Scholar]
- Bartus K, James ND, Bosch KD, Bradbury EJ. Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol. 2012;235(1):5–17. doi: 10.1016/j.expneurol.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14(23):2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Blesch A, Yang H, Weidner N, Hoang A, Otero D. Axonal responses to cellularly delivered NT-4/5 after spinal cord injury. Mol Cell Neurosci. 2004;27(2):190–201. doi: 10.1016/j.mcn.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda) 2005;20(1):70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88(6):1182–1192. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci. 2002;15(4):613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- Bretzner F, Liu J, Currie E, Roskams AJ, Tetzlaff W. Undesired effects of a combinatorial treatment for spinal cord injury—transplantation of olfactory ensheathing cells and BDNF infusion to the red nucleus. Eur J Neurosci. 2008;28(9):1795–1807. doi: 10.1111/j.1460-9568.2008.06462.x. [DOI] [PubMed] [Google Scholar]
- Brock JH, Rosenzweig ES, Blesch A, Moseanko R, Havton LA, Edgerton VR, Tuszynski MH. Local and remote growth factor effects after primate spinal cord injury. J Neurosci. 2010;30(29):9728–9737. doi: 10.1523/JNEUROSCI.1924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SRy. Degeneration and regeneration of the nervous system. Hafner; New York: 1928. [Google Scholar]
- Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci. 2006;26(11):2923–2932. doi: 10.1523/JNEUROSCI.4390-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25(30):6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Cosgaya JM, Wu YJ, Shooter EM. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc Natl Acad Sci USA. 2001;98(25):14661–14668. doi: 10.1073/pnas.251543398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Watkins TA, Cosgaya JM, Zhang CZ, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43(2):183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003a;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003b;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chiaretti A, Antonelli A, Genovese O, Pezzotti P, Rocco CD, Viola L, Riccardi R. Nerve growth factor and doublecortin expression correlates with improved outcome in children with severe traumatic brain injury. J Trauma. 2008;65(1):80–85. doi: 10.1097/TA.0b013e31805f7036. [DOI] [PubMed] [Google Scholar]
- Chu Q, Wang Y, Fu X, Zhang S. Mechanism of in vitro differentiation of bone marrow stromal cells into neuron-like cells. J Huazhong Univ Sci Technolog Med Sci. 2004;24(3):259–261. doi: 10.1007/BF02832006. [DOI] [PubMed] [Google Scholar]
- Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298(5596):1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21(23):9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deumens R, Koopmans GC, Joosten EA. Regeneration of descending axon tracts after spinal cord injury. Prog Neurobiol. 2005;77(1–2):57–89. doi: 10.1016/j.pneurobio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. J Neurol Sci. 2005;233(1–2):43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Epa WR, Markovska K, Barrett GL. The p75 neurotrophin receptor enhances TrkA signalling by binding to Shc and augmenting its phosphorylation. J Neurochem. 2004;89(2):344–353. doi: 10.1111/j.1471-4159.2004.02344.x. [DOI] [PubMed] [Google Scholar]
- Ferguson IA, Koide T, Rush RA. Stimulation of corticospinal tract regeneration in the chronically injured spinal cord. Eur J Neurosci. 2001;13(5):1059–1064. doi: 10.1046/j.1460-9568.2001.01482.x. [DOI] [PubMed] [Google Scholar]
- Ferraro GB, Alabed YZ, Fournier AE. Molecular targets to promote central nervous system regeneration. Curr Neurovasc Res. 2004;1(1):61–75. doi: 10.2174/1567202043480251. [DOI] [PubMed] [Google Scholar]
- Freidman WJ. Proneurotrophin, seizures, and neuronal apoptosis. Neuroscienctist. 2010;16(3):244–252. doi: 10.1177/1073858409349903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey CM, Kwok JCF, Carulli D, Rhodes KE, Fawcett JW. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur J Neurosci. 2008;27(6):1373–1390. doi: 10.1111/j.1460-9568.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- Gámez E, Ikezaki K, Fukui M, Matsuda T. Photoconstructs of nerve guidance prosthesis using photoreactive gelatin as a scaffold. Cell Transplant. 2003;12(5):481–490. doi: 10.3727/000000003108747046. [DOI] [PubMed] [Google Scholar]
- Grill RJ, Blesch A, Tuszynski MH. Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp Neurol. 1997;148(2):444–452. doi: 10.1006/exnr.1997.6704. [DOI] [PubMed] [Google Scholar]
- Hendriks WT, Ruitenberg MJ, Blits B, Boer GJ, Verhaagen J. Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog Brain Res. 2004;146:451–476. doi: 10.1016/S0079-6123(03)46029-9. [DOI] [PubMed] [Google Scholar]
- Höke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26(38):9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Jamshidi P, Löw K, Blesch A, Tuszynski MH. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc Natl Acad Sci USA. 2009;106(17):7215–7220. doi: 10.1073/pnas.0810624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Tuszynski MH. Neurotrophins: potential therapeutic tools for the treatment of spinal cord injury. Neurotherapeutics. 2011;8(4):694–703. doi: 10.1007/s13311-011-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72(1):609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Iarikov DE, Kim BG, Dai HN, McAtee M, Kuhn PL, Bregman BS. Delayed transplantation with exogenous neurotrophin administration enhances plasticity of corticofugal projections after spinal cord injury. J Neurotrauma. 2007;24(4):690–702. doi: 10.1089/neu.2006.0172. [DOI] [PubMed] [Google Scholar]
- Ide C. Peripheral nerve regeneration. Neurosci Res. 1996;25(2):101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- Jin Y, Ziemba KS, Smith GM. Axon growth across a lesion site along a preformed guidance pathway in the brain. Exp Neurol. 2008;210(2):521–530. doi: 10.1016/j.expneurol.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003;23(28):9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64(2):165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Choe Y, Park J, Cho S, Kim K. Activation of protein kinase A induces neuronal differentiation of HiB5 hippocampal progenitor cells. Brain Res Mol Brain Res. 2002;109(1–2):134–145. doi: 10.1016/S0169-328X(02)00550-8. [DOI] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44(3):439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci. 1997;17(24):9583–9595. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118(2):243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Kusano K, Enomoto M, Hirai T, Tsoulfas P, Sotome S, Shinomiya K, Okawa A. Transplanted neural progenitor cells expressing mutant NT3 promote myelination and partial hindlimb recovery in the chronic phase after spinal cord injury. Biochem Biophys Res Commun. 2010;393(4):812–817. doi: 10.1016/j.bbrc.2010.02.088. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Lam C, Plunet W, Oschipok LW, Hauswirth W, Di Polo A, Blesch A, Tetzlaff W. Brain-derived neurotrophic factor gene transfer with adeno-associated viral and lentiviral vectors prevents rubrospinal neuronal atrophy and stimulates regeneration-associated gene expression after acute cervical spinal cord injury. Spine. 2007;32(11):1164–1173. doi: 10.1097/BRS.0b013e318053ec35. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci USA. 2002;99(5):3246–3251. doi: 10.1073/pnas.052308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci USA. 2010;107(8):3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann HC, Höke A. Schwann cells as a therapeutic target for peripheral neuropathies. CNS Neurol Disord Drug Targets. 2010;9(6):801–806. doi: 10.2174/187152710793237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69(5):341–374. doi: 10.1016/S0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Longhi L, Watson DJ, Saatman KE, Thompson HJ, Zhang C, Fujimoto S, Royo N, Castelbuono D, Raghupathi R, Trojanowski JQ, Lee VM, Wolfe JH, Stocchetti N, McIntosh TK. Ex vivo gene therapy using targeted engraftment of NGF-expressing human NT2N neurons attenuates cognitive deficits following traumatic brain injury in mice. J Neurotrauma. 2004a;21(12):1723–1736. doi: 10.1089/neu.2004.21.1723. [DOI] [PubMed] [Google Scholar]
- Longhi L, Watson DJ, Saatman KE, Thompson HJ, Zhang C, Fujimoto S, Royo N, Castelbuono D, Raghupathi R, Trojanowski JQ, Lee VM, Wolfe JH, Stocchetti N, McIntosh TK. Ex vivo gene therapy using targeted engraftment of NGF-expressing human NT2N neurons attenuates cognitive deficits following traumatic brain injury in mice. J Neurotrauma. 2004b;21(12):1723–1736. doi: 10.1089/neu.2004.21.1723. [DOI] [PubMed] [Google Scholar]
- Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS ONE. 2011;6(3):e17899. doi: 10.1371/journal.pone.0017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6(8):603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Lu P, Blesch A, Tuszynski MH. Neurotrophism without neurotropism: BDNF promotes survival but not growth of lesioned corticospinal neurons. J Comp Neurol. 2001;436(4):456–470. doi: 10.1002/cne.1080. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115–129. doi: 10.1016/S0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Wang L, Chopp M. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J Neurotrauma. 2002;19(12):1609–1617. doi: 10.1089/089771502762300265. [DOI] [PubMed] [Google Scholar]
- Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NGF, Silver J, Onifer SM. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2008;209(2):426–445. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson JL, Strong MK, Steward O. A reassessment of whether cortical motor neurons die following spinal cord injury. J Comp Neurol. 2011;519(14):2852–2869. doi: 10.1002/cne.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova LN, Novikov LN, Kellerth JO. Survival effects of BDNF and NT-3 on axotomized rubrospinal neurons depend on the temporal pattern of neurotrophin administration. Eur J Neurosci. 2000;12(2):776–780. doi: 10.1046/j.1460-9568.2000.00978.x. [DOI] [PubMed] [Google Scholar]
- Philips MF, Mattiasson G, Wieloch T, Björklund A, Johansson BB, Tomasevic G, Martínez-Serrano A, Lenzlinger PM, Sinson G, Grady MS, McIntosh TK. Neuroprotective and behavioral efficacy of nerve growth factor-transfected hippocampal progenitor cell transplants after experimental traumatic brain injury. J Neurosurg. 2001;94(5):765–774. doi: 10.3171/jns.2001.94.5.0765. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403(6767):312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002;17(4):1137–1152. doi: 10.14670/HH-17.1137. [DOI] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci. 2001;21(21):8408–8416. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MI, Smith GM. Adenoviral gene transfer into the normal and injured spinal cord: enhanced transgene stability by combined administration of temperature-sensitive virus and transient immune blockade. Gene Ther. 1998;5(12):1612–1621. doi: 10.1038/sj.gt.3300774. [DOI] [PubMed] [Google Scholar]
- Royo NC, Schouten JW, Fulp CT, Shimizu S, Marklund N, Graham DI, McIntosh TK. From cell death to neuronal regeneration: building a new brain after traumatic brain injury. J Neuropathol Exp Neurol. 2003;62(8):801–811. doi: 10.1093/jnen/62.8.801. [DOI] [PubMed] [Google Scholar]
- Sinson G, Voddi M, McIntosh TK. Combined fetal neural transplantation and nerve growth factor infusion: effects on neurological outcome following fluid-percussion brain injury in the rat. J Neurosurg. 1996;84(4):655–662. doi: 10.3171/jns.1996.84.4.0655. [DOI] [PubMed] [Google Scholar]
- Smith GM, Onifer S. Construction of pathways to promote axon growth within the adult central nervous system. Brain Research Bulletin Brain Res Bull. 2011;84(4–5) doi: 10.1016/j.brainresbull.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Romero MI. Adenoviral-mediated gene transfer to enhance neuronal survival, growth, and regeneration. J Neurosci Res. 1999;55(2):147–157. doi: 10.1002/(SICI)1097-4547(19990115)55:2<147::AID-JNR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Cai J, Nelson KD, Peng XJ, Smith GM. Functional repair after dorsal root rhizotomy using nerve conduits and neurotrophic molecules. Eur J Neurosci. 2004a;20(5):1211–1218. doi: 10.1111/j.1460-9568.2004.03595.x. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004b;24(4):819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113(3):226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias CA, Shumsky JS, Shibata M, Tuszynski MH, Fischer I, Tessler A, Murray M. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol. 2003;184(1):97–113. doi: 10.1016/S0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- Tonra JR, Curtis R, Wong V, Cliffer KD, Park JS, Timmes A, Nguyen T, Lindsay RM, Acheson A, DiStefano PS. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18(11):4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Kleppner SR, Hartley RS, Miyazono M, Fraser NW, Kesari S, Lee VM. Transfectable and transplantable postmitotic human neurons: a potential “platform” for gene therapy of nervous system diseases. Exp Neurol. 1997;144(1):92–97. doi: 10.1006/exnr.1996.6393. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A. Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp Neurol. 1996;137(1):157–173. doi: 10.1006/exnr.1996.0016. [DOI] [PubMed] [Google Scholar]
- Vavrek R, Girgis J, Tetzlaff W, Hiebert GW, Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129(Pt 6):1534–1545. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Yao WF, Deng QJ, Zhang XH, Zhang JN. Protective effects of BDNF overexpression bone marrow stromal cell transplantation in rat models of traumatic brain injury. J Mol Neurosci. 2013;49(2):409–416. doi: 10.1007/s12031-012-9908-0. [DOI] [PubMed] [Google Scholar]
- Woolley AG, Tait KJ, Hurren BJ, Fisher L, Sheard PW, Duxson MJ. Developmental loss of NT-3 in vivo results in reduced levels of myelin-specific proteins, a reduced extent of myelination and increased apoptosis of Schwann cells. Glia. 2008;56(3):306–317. doi: 10.1002/glia.20614. [DOI] [PubMed] [Google Scholar]
- Xiao J, Wong A, Kilpatrick T, Murray S. BDNF ENHANCES CENTRAL NERVOUS SYSTEM MYELINATION VIA A DIRECT SIGNALLING TO OLIGODENDROGLIAL TrKB RECEPTORS. J Neurochem. 2010;115:36–36. [Google Scholar]
- Xiao JH, Kilpatrick TJ, Murray SS. The role of neurotrophins in the regulation of myelin development. Neurosignals. 2009;17(4):265–276. doi: 10.1159/000231893. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guénard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134(2):261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Ye JH, Houle JD. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp Neurol. 1997;143(1):70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Li WP, Zhou FH, Zhong JH, Mi JX, Wu LL, Xian CJ. Differential effects of endogenous brain-derived neurotrophic factor on the survival of axotomized sensory neurons in dorsal root ganglia: a possible role for the p75 neurotrophin receptor. Neuroscience. 2005;132(3):591–603. doi: 10.1016/j.neuroscience.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Chen H, Zhang K, Yang H, Liu J, Huang Q. Protective effect of nerve growth factor on neurons after traumatic brain injury. J Basic Clin Physiol Pharmacol. 2003;14(3):217–224. doi: 10.1515/JBCPP.2003.14.3.217. [DOI] [PubMed] [Google Scholar]
- Zou LL, Huang L, Hayes RL, Black C, Qiu YH, Perez-Polo JR, Le W, Clifton GL, Yang K. Liposome-mediated NGF gene transfection following neuronal injury: potential therapeutic applications. Gene Ther. 1999;6(6):994–1005. doi: 10.1038/sj.gt.3300936. [DOI] [PubMed] [Google Scholar]


